Received: Thu 03, Sep 2020
Accepted: Tue 22, Sep 2020
Abstract
Karolinska University Hospital has experience of treating patients with (TTFields, Optune®) since 2014. This case report presents a 22-year-old female patient with diffuse midline glioma, H3-K27M mutated, WHO Grade IV. The tumor was unresponsive to standard of care. The patient experienced even severe cytostatic-induced hematological complication and severe fatigue that markedly reduced her quality of life. TTFields were initiated. So far, at 3 years, there are no signs of progression, radiologic evaluation shows tumor regression (> 80%). The patient is continuing treatment with maintained quality of life.
1. Introduction
Diffuse midline glioma, H3K27M, WHO grade IV, is a rare tumor, mainly in children and adolescents. The prognosis in adults may be slightly better than in children, but it is more aggressive and treatment-resistant than glioblastoma [1-3]. According to WHO (World Health Organization) classification, midline glioma has its own tumor entity [4, 5]; the tumor growth is diffuse and infiltrates the midline and brainstem and has the characteristic mutation H3K27M [5]. There is no established consensus of therapy for these patients. In Sweden, it is practice that the patients are treated in the same way as glioblastoma [6].
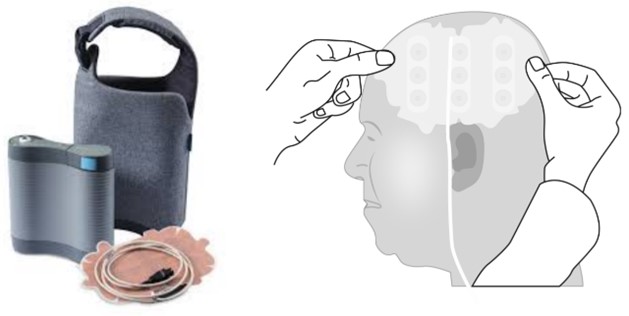
Tumor Treating Fields (Optune®) is a loco-regional non-invasive therapy that has documented antitumoral inhibition and is registered as a complementary therapy for adult patients with glioblastoma WHO grade IV after radiation therapy, in combination with adjuvant temozolomide [6-8]. TTFields act through arrays placed on the scalp (Figure 1), connected to a portable unit that generates alternative electric fields of low intensity, 1-3 V/cm, and a frequency of 200 kHz. The generated electric fields inhibit cell division by interfering with the mitotic process [9-11]. A case report describes TTFields treatment in children with midline glioma [12]. Our report is the first adult with diffuse midline glioma, H3K27M mutated that received TTFields therapy.
2. Case Report
A 22-year-old female student, previously healthy, presented with 2 months headache, and was admitted to the emergency care as a stroke alarm in June 2017 because of abrupt worsening of symptoms, speech difficulty, vomiting, right-sided weakness and a generalized seizure. The MRI of the brain showed a central tumor, in the left thalamus and upper pons (4x6x5 cm), heterogeneous, partly calcified, diffusely infiltrative, cystic, with expansive effect (Figure 2). In reason of the localization, only an open biopsy was possible in July 2017. The pathological analysis showed a diffuse midline glioma, with Isocitrate Dehydrogenase wild type (IDH1 wt), no methylation of O(6)-methylguanine-DNA methyl-transferase (MGMT unmethylated), WHO grade IV, H3K27M mutation. The patient received radio-chemotherapy (total 60 Gy with concomitant temozolomide), without complication. Already 4 weeks after terminated radiation therapy, the patient developed acute headache.
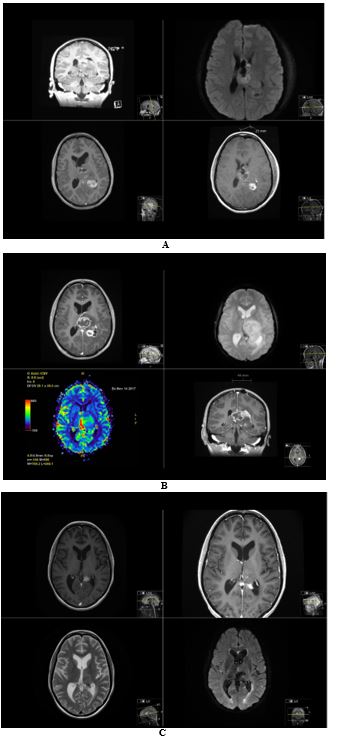
The MRI showed increased tumor volume, increased contrast enhancement with gadolinium, restricted diffusion-weighted imaging (DWI) and increased cerebral blood perfusion (rCBV) in the tumor. This was therefore considered as a true progression rather than radiation-induced pseudo progression. The patient was then treated with lomustine (CCNU, 120mg/m2, once q 42 days), but rapidly demonstrated bad tolerance to chemotherapy (leucopenia, neutropenia) and increased fatigue (bedridden most of the day) and severe memory difficulties. She was then started on TTFields in October 2017 as part of a compassionate use program from the supplier, Novocure. The lomustine was definitively stopped after the 2nd cycle (chronic leucopenia, and fatigue). The patient developed 6 months later a secondary hemophagocytic lymfohistiocytosis (HLH) which was life threatening but recovered after intensive care. She was no candidate to more chemotherapy.
The patient described the change in her life after chemotherapy and under TTFields, as “day and night”, especially considering the fatigue. The patient reported no side effects of TTFields, and her quality of life increased steadily. The average time on TTFields is 90% daily, she can exercise, practice hobbies (pottery, climbing), and will now again start her studies, since even memory difficulties partially improved.
3. Discussion
This case report presents a unique description of the long-term results of TTFields therapy in an adult patient with midline glioma H3K27M mutated. Besides the objective tumor response, there was a positive impact on the quality of life.
Since the tumor had unmethylated MGMT, a dismal prognosis was expected as well as a poor response to chemotherapy. An interesting observation was the dramatic improvement in the quality of life after chemotherapy ceased and the patient’s positive attitude to TTFields as self-care, where she could have control. The effect of radiation therapy is notoriously difficult to assess weeks to months after ending treatment [13], the clinical symptoms and radiological work-up pointed strongly towards a therapy-resistant disease. A possible spontaneous regression of tumor volume has been described in children with other tumor types and milder tumor biology [14, 15], however the successive objective response after TTFields is consistent with a genuine response to therapy. The radiologic response in TTFields therapy is slow according to its mechanism of action with a median time of 5, 2 months [16]. Patients receiving TTFields monotherapy with objective radiologic response also have a better overall survival [16]. Higher compliance with TTFields (> 75% daily use, 18 hours per day) is directly correlated to improved overall survival [17, 18]. This is explained by the fact that TTFields has no half-time and works only when “on”. Patients with newly diagnosed glioblastoma with an average daily use of TTFields > 90% showed a 5-year overall survival of 29% [18].
4. Conclusion
TTFields is registered in several countries and used in the clinical routine for patients with Glioblastoma. Evidence generation for patients with H3K27-mutant Diffuse Midline Glioma is difficult because of the rare nature of the disease and low incidence in adults. This report will point out the importance of individual assessment in the treatment plan of diffuse midline glioma, the available standard therapy for glioblastoma, and TTFields as a potential alternative treatment option.
Conflicts of Interest
None.
Acknowledgments
To Alex Gholiha, MD for editorial support and review of the manuscript, and to Novocure (Sweden) for providing (Figure 1), and the compassionate use program that made it possible for this patient to receive TTFields treatment.
REFERENCES
- Daoud EV, Rajaram V, Cai C, et al. “Adult Brainstem Gliomas With H3K27M Mutation: Radiology, Pathology, and Prognosis.” J Neuropathol Exp Neurol, vol. 77, no. 4, pp. 302-311, 2018. View at: Publisher Site | PubMed
- Schreck KC, Ranjan S, Skorupan N, et al. “Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas.” J Neurooncol, vol. 143, no. 1, pp. 87-93, 2019. View at: Publisher Site | PubMed
- Meyronet D, Esteban Mader M, Bonnet C, et al. “Characteristics of H3 K27M-mutant gliomas in adults.” Neuro Oncol, vol. 19, no. 8, pp. 1127-1134, 2017. View at: Publisher Site | PubMed
- Louis DN, Perry A, Reifenberger G, et al. “The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary.” Acta Neuropathol, vol. 131, no. 6, pp. 803-820, 2016. View at: Publisher Site | PubMed
- Louis DN, Giannini C, Capper D, et al. “cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant.” Acta Neuropathol, vol. 135, no. 4, pp. 639-642, 2018. View at: Publisher Site | PubMed
- Regional cancercentrum i samverkan Nationellt vårdprogram “Tumörer i hjärna och ryggmärg.” 2020.
- Landstingens samverkan modell för läkemedel. “Optune för behandling av glioblastom.” NT-rådets yttrande till landstingen, 2018.
- Stupp R, Taillibert S, Kanner A, et al. “Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial.” JAMA, vol. 318, no. 23, pp. 2306-2316, 2017. View at: Publisher Site | PubMed
- Giladi M, Schneiderman RS, Voloshin T, et al. “Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells.” Sci Rep, vol. 5, pp. 18046, 2015. View at: Publisher Site | PubMed
- Kirson ED, Dbalý V, Tovarys F, et al. “Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors.” Proc Natl Acad Sci U S A, vol. 104, no. 24, pp. 10152-10157, 2007. View at: Publisher Site | PubMed
- Hottinger AF, Pacheco P, Stupp R “Tumor treating fields: a novel treatment modality and its use in brain tumors.” Neuro Oncol, vol. 18, no. 10, pp. 1338-1349, 2016. View at: Publisher Site | PubMed
- Toledano H, Abebe Campino G, Dvir R, et al. “P01.085 Experience with TTFields (Optune®) in pediatric high grade glioma patients in Israel.” Neuro-Oncology, vol. 20, no. 3, pp. iii249-iii250, 2018. View at: Publisher Site
- Baskar R, Lee KA, Yeo R, et al. “Cancer and radiation therapy: current advances and future directions.” Int J Med Sci, vol. 9, no. 3, pp. 193-199, 2012. View at: Publisher Site | PubMed
- Lenard HG, Engelbrecht V, Janssen G, et al. “Complete remission of a diffuse pontine glioma.” Neuropediatrics, vol. 29, no. 6, pp. 328-330, 1998. View at: Publisher Site | PubMed
- Ishihara M, Yamamoto K, Miwa H, et al. “Spontaneous complete regression of a brain stem glioma pathologically diagnosed as a high-grade glioma.” Childs Nerv Syst, vol. 33, no. 12, pp. 2177-2180, 2017. View at: Publisher Site | PubMed
- Vymazal J, Wong ET “Response patterns of recurrent glioblastomas treated with tumor-treating fields.” Semin Oncol, vol. 41, no. 6, pp. S14-S24, 2014. View at: Publisher Site | PubMed
- Kanner AA, Wong ET, Villano JL, et al. “Post Hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100ATM system versus best physician’s choice chemotherapy.” Semin Oncol, vol. 41, no. 6, pp. S25-S34, 2014. View at: Publisher Site | PubMed
- Toms SA, Kim CY, Nicholas G, et al. “Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial.” J Neurooncol, vol. 141, no. 2, pp. 467-473, 2019. View at: Publisher Site | PubMed
- Stupp R, Wong ET, Kanner AA, et al. “NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality.” Eur J Cancer, vol. 48, no. 14, pp. 2192-2202, 1990. View at: Publisher Site | PubMed
