Received: Tue 21, Jul 2020
Accepted: Tue 11, Aug 2020
Abstract
Objective: To review the key points and limitations of potentiating autologous split-thickness skin graft survival using fibrin glue for the reconstruction of cutaneous defects following oncologic resection of facial tumors.
Case Report: A patient who underwent resection of a facial fibrosarcoma and subsequent reconstruction by autologous split-thickness skin graft secured with biologic fibrin glue.
Results: There was ninety-percent primary survival of the skin graft at three weeks. Partial necrosis of 10% of the graft surface was successfully managed by debridement and topical application of honey.
Conclusion: The time-honored autologous split-thickness skin graft is a useful means of reconstructing cutaneous defects after resection of facial tumors. The application of fibrin glue may avoid the conventional bolster dressing used to stabilize the graft during the early postoperative period.
1. Introduction
Cutaneous defects of the face following oncologic resections present several challenges to the reconstructive surgeon. Local flaps can provide vascularized tissue with favorable colour, thickness, contour match, and the ability to preserve aesthetic subunits [1]. When regional flaps are contraindicated, autologous skin grafts are suitable alternatives. Though free skin grafting may have been successfully performed in India by the Koomas caste of brick and tilemakers as early as the 6th century BCE, modern successful autologous skin transfer traces back to work by Cooper, Wolfe, Reverdin, and Le Fort in the 19th century [2, 3]. Ollier and Thiersch further refined this technique with the introduction of split-thickness grafts, where the dermal component of the skin is reduced [4]. The incorporation and survival of the graft, or graft take, depends on the restoration of blood flow to the ischaemic donor tissue [5, 6]. Direct apposition between the donor skin and the recipient wound bed facilitates plasmatic imbibition, inosculation, revascularization, and enhances graft take.
Various techniques to improve graft adherence and limit hematoma or seroma formation have been described, including the use of pressure dressing and negative-pressure wound therapy [7-9]. Biologic fibrin glues have been applied to support skin graft survival since World War II [10, 11]. Since early reports, multiple studies have shown that fibrin glues can reduce operative times, hematoma risk, need for negative-pressure wound therapy and bolsters, with equivalent graft survival rates [12-15]. Herein, we describe the successful use of biologic fibrin glue with split-thickness skin graft reconstruction of a cutaneous facial defect.
2. Materials and Methods
A PubMed search conducted on January 1, 2020 with the search-terms “face” and “skin graft” retrieved more than 5,300 articles, with several promoting various local or locoregional flaps for reconstruction of skin defects after resection of facial tumors. Although autologous free skin graft was preferred less overall, in one study it accounted for 22.3% of reconstructions between 2010 and 2014 in a plastic and reconstructive surgery department [16]. The present study revisits the key benefits and limitations of this time-honored technique and the novel application of biologic fibrin glue to improve graft take in head and neck reconstruction.
3. Case Report
Mr. A, aged 85 years, presented with a two-month history of a cutaneous mass adjacent to the left zygomatic arch (Figure 1A). An initial biopsy suggested a histologic diagnosis of atypical fibroxanthoma. The patient’s medical history also included: an ipsilateral parotid adenoid cystic carcinoma treated 9 years prior by a facial nerve-sparing parotidectomy and ipsilateral II-III neck dissection complicated by a local recurrence after 13 months treated by surgical resection and 65 Gray adjuvant radiation therapy; prostate adenocarcinoma treated by radiation therapy 24 months prior to presentation; and, high blood pressure. Physical examination revealed an isolated ulcerated temporal skin lesion, (Figure 1A) mobile, without facial nerve palsy or palpable head and neck lymphadenopathy. The tumor measure 25 mm in diameter on MRI, without evidence of underlying muscular or bony infiltration. No pathologic lymphadenopathy was seen, nor evidence of recurrence of the adenoid cystic carcinoma at the ipsilateral parotid.
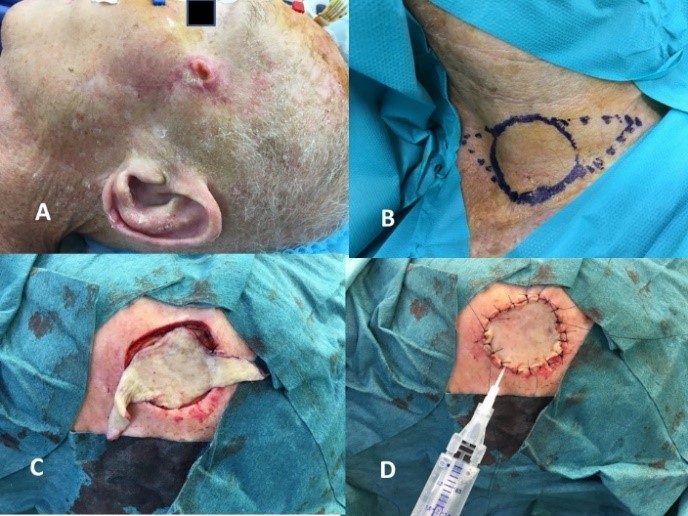
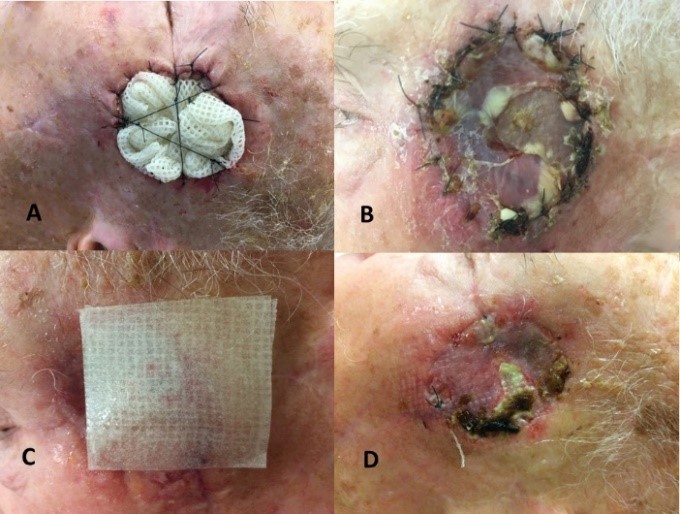
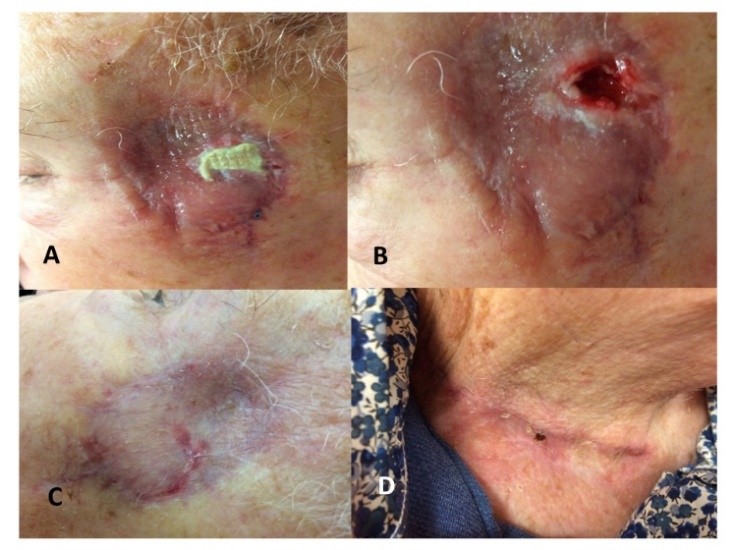
Our institutional multidisciplinary tumor board recommended resection by Mohs surgery [17] with autologous free skin graft reconstruction. The mass was excised under facial nerve neuromonitoring, resulting in a 5 cm defect (Figure 1C). Once adequate margins and hemostasis had been achieved, a skin graft was harvested from the contralateral lower neck (Figure 1B). The graft was thinned on the dermal side and secured to the edges of the defect using interrupted Crinercé® 4/0 sutures (Figure 1D). Without perforating the graft, a syringe of Tisucol® biologic fibrin glue was injected between the skin sutures (Figure 1D). Light manual pressure was applied on the graft for a few seconds, and the excess glue was removed through the suture line. Finally, a Jelonet® bolster dressing (Figure 2A) was applied for 7 days. After removing the bolster graft pain was noted (Figure 2B) and local honey-based dressing [18] was applied daily for 3 weeks (Figure 2C). At 3 weeks, an 8 x 5mm area skin necrosis was debrided, representing 10% of the initial graft area (Figures 2D, 3A & 3B). Honey-impregnated dressings were applied for an additional 3 weeks. The status of the skin-graft was photodocumented at 3 weeks and 3 months postoperatively (Figures 3B & 3C). The final pathologic diagnosis was fibrosarcoma with negative superficial and deep margins (R0). No adjuvant treatment was recommended. The patient was disease free at 6 months follow-up.
4. Discussion
Reconstruction of facial defects resulting from resection of malignant tumors by Mohs microsurgery [17] is best accomplished with either local or regional flaps adapted to the anatomic site and extent of resection. When such flaps are difficult to implement due to comorbidities and/or risk of functional injury, autologous free skin graft transfer is a valuable alternative [16, 19-23]. In the case of Mr. A, his prior ipsilateral parotidectomy, exposure of the perilesional tissues to radiation therapy, and potential risk of damage to facial nerve branches were relative contraindications to locoregional flap reconstruction. The time-honored autologous skin graft was considered to be the best first-line surgical option.
The success of free skin transfer depends on donor site, recipient site and technical factors [16, 19-23]. The choice of the skin donor site should be guided by the recipient site. In our experience a retro-auricular skin graft donor site is preferred for small defects, but for larger wounds, such as the present case (Figures 1A & 1C), skin harvest from the lower neck (Figure 1B) provides a better approximation of the colour and sebaceous quality of the skin surrounding the resection area, with minimal donor-site morbidity (Figure 3D). Recipient site hemostasis must be painstakingly achieved, with minimal cauterization, to augment deep revascularization and limit the risk hematoma and graft necrosis. The underlying tissue must be favorable for the graft to take. Prior irradiation, underlying bone and cartilage without vascularized periosteum/perichondrium, local infection negatively impact graft survival. In case of active infection, antibiotic therapy targeting Streptococcus pyogenes and delayed reconstruction are recommended.
In terms of technique, the graft needs to be carefully thinned of adipose tissue on the dermal aspect and must be sutured into place without tension at the recipient bed (Figure 1C). To limit tension from movement and shear stress that would jeopardize graft survival, a bolster dressing (Figure 2A) is recommended by several authors [20, 23]. However, a recent literature review by the University of Colorado [24] cast doubt on this long-standing practice, suggesting that such dressings may offer little benefit for graft integration and survival, rate of complications or aesthetic outcome. In their experience, the use of a bolster dressing being should be left to the surgeon’s discretion. In our case, though a bolster was applied (Figure 2B), it appeared to us that the injection of fibrin glue (Figure 1D) was likely sufficient to achieve the stability of the graft. Simulating the exudative phase of healing, biologic fibrin glue promotes graft adherence to the underlying tissue by the catalyzation of a fibrin polymer from the reaction between the fibrinogen and calcium present in the glue [25, 26]. The polymer promotes the growth of collagen-producing fibroblasts while reducing the degradation of the fibrin deposited by the blood in skin wounds. Fibrin deposits are maintained by local tissue fibroblasts and by the migration of additional fibroblasts to the wound, which produce procollagen (type-I collagen). The fibroblasts move around the wound site using fibrin and fibronectin, promoting healing by contributing to the underlying local molecular matrix.
Finally, our case demonstrates the importance of local wound care, including the beneficial impact of honey (Figures 3 & 4). The partial necrosis of 10% of the graft (Figure 3A), responded well to the local application of honey-impregnated dressings [18], resulting in an acceptable aesthetic outcome with no significant discrepancy in thickness or colour between the graft and surrounding recipient tissue (Figure 3C).
Conflicts of Interest
None.
Funding
None.
Acknowledgments
The authors would like to thank the Association Progrès 2000 for technical support.
REFERENCES
- Ilankovan V, Ethunandan M, Seah TE “Decision-Making Process.” Local Flaps in Facial Reconstruction: A Defect Based Approach, Cham: Springer International Publishing, pp. 63-64, 2015. View at: Publisher Site
- Hauben DJ, Baruchin A, Mahler A “On the history of the free skin graft.” Ann Plast Surg, vol. 9, no. 3, pp 242-245. View at: Publisher Site | PubMed
- Cohen J “Earliest attempt at free skin grafting.” Ann Plast Surg, vol. 34, no. 5, pp. 552-553, 1995. View at: Publisher Site | PubMed
- Singh M, Nuutila K, Collins KC, et al. “Evolution of skin grafting for treatment of burns: Reverdin pinch grafting to Tanner mesh grafting and beyond.” Burns, vol. 43, no. 6, pp. 1149-1154, 2017. View at: Publisher Site | PubMed
- Haller JA Jr, Billingham RE “Studies of the origin of the vasculature in free skin grafts.” Ann Surg, vol. 166, no. 6, pp. 896-901, 1967. View at: Publisher Site | PubMed
- Hira M, Tajima S “Biochemical Study on the Process of Skin Graft Take.” Ann Plast Surg, vol. 29, no. 1, pp. 47-54, 1992. View at: Publisher Site | PubMed
- Isago T, Nozaki M, Kikuchi Y, et al. “Skin graft fixation with negative-pressure dressings.” J Dermatol, vol. 30, no. 9, pp. 673-678, 2003. View at: Publisher Site | PubMed
- Kim SW, Kim JH, Kim JT, et al. “A simple and fast dressing for skin grafts: comparison with traditional techniques.” J Wound Care, vol. 27, no. 7, pp. 417-420, 2018. View at: Publisher Site | PubMed
- Shen X, Zhan T, Wei D, et al. “Comparison of Efficacy and Complications Between Negative Pressure Wound Therapy and Conventional Mechanical Fixation in Skin Grafts: A Retrospective Analysis.” Wounds, vol. 31, no. 8, pp. 213-218, 2019. View at: PubMed
- Cronkite EP, Lozner EL, Deaver JM “Use of Thrombin and Fibrinogen in Sking Grafting: preliminary report.” JAMA, vol. 124, pp. 976-978, 1944. View at: Publisher Site
- Tidrick RT, Warner ED “Fibrin fixation of skin transplants.” Surg, vol. 15, pp. 90-95, 1944. View at: Publisher Site
- Grunzweig KA, Ascha M, Kumar AR “Fibrin tissue sealant and minor skin grafts in burn surgery: A systematic review and meta-analysis.” J Plast Reconstr Aesthet Surg, vol. 72, no. 6, pp. 871-883, 2019. View at: Publisher Site | PubMed
- Miller R, Wormald JCR, Wade RG, et al. “Systematic review of fibrin glue in burn wound reconstruction.” Br J Surg, vol. 106, no. 3, pp. 165-173, 2019. View at: Publisher Site | PubMed
- Mullens CL, Messa CA, Kozak GM, et al. “To Glue or Not to Glue? Analysis of Fibrin Glue for Split-thickness Skin Graft Fixation.” Plast Reconstr Surg Glob Open, vol. 7, no. 5, pp. e2187, 2019. View at: Publisher Site | PubMed
- Reddy KS, Chittoria RK, Babu P, et al. “Effectiveness of Fibrin Glue in Adherence of Skin Graft.” J Cutan Aesthet Surg, vol. 10, no. 2, pp. 72-75, 2017. View at: Publisher Site
- Lee KS, Kim JO, Kim NG, et al. “A Comparison of the Local Flap and Skin Graft by Location of Face in Reconstruction after Resection of Facial Skin Cancer.” Arch Craniofac Surg, vol. 18, no. 4, pp. 255-260, 2017. View at: Publisher Site
- Mohs FE “Chemosurgery: microscopically controlled surgery for skin cancer--past, present and future.” J Dermatol Surg Oncol, vol. 4, no. 1, pp. 41-54, 1978. View at: Publisher Site | PubMed
- Werner A, Laccourreye O “Honey in otorhinolaryngology: when, why and how?” Eur Ann Otorhinolaryngol Head Neck Dis, vol. 128, no. 3, pp. 133-137, 2011. View at: Publisher Site | PubMed
- Ramsey ML, Walker B, Patel BC “Full Thickness Skin Grafts.” StatPearls, Treasure Island (FL): StatPearls Publishing, 2020.
- Olson MD, Hamilton GS “Scalp and Forehead Defects in the Post-Mohs Surgery Patient.” Facial Plast Surg Clin North Am, vol. 25, no. 3, pp. 365-375, 2017. View at: Publisher Site | PubMed
- Rustemeyer J, Thieme V, Günther L, et al. “Experiences with surgical management of facial basal cell carcinoma and procedures for plastic reconstruction.” Mund Kiefer Gesichtschir, vol. 9, no. 4, pp. 220-224, 2005. View at: Publisher Site | PubMed
- Sapthavee A, Munaretto N, Toriumi DM “Skin Grafts vs Local Flaps for Reconstruction of Nasal Defects: A Retrospective Cohort Study.” JAMA Facial Plast Surg, vol. 17, no. 4, pp. 270-273, 2015. View at: Publisher Site | PubMed
- Ratner D “Skin grafting.” Semin Cutan Med Surg, vol. 22, no. 4, pp. 295-305, 2003. View at: Publisher Site | PubMed
- Kromka W, Cameron M, Fathi R “Tie-Over Bolster Dressings vs Basting Sutures for the Closure of Full-Thickness Skin Grafts: A Review of the Literature.” J Cutan Med Surg, vol. 22, no. 6, pp. 602-606, 2018. View at: Publisher Site | PubMed
- Currie LJ, Sharpe JR, Martin R “The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review.” Plast Reconstr Surg, vol. 108, no. 6, pp. 1713-1726, 2001. View at: Publisher Site | PubMed
- Foster K, Greenhalgh D, Gamelli RL, et al. “Efficacy and safety of a fibrin sealant for adherence of autologous skin grafts to burn wounds: results of a phase 3 clinical study.” J Burn Care Res, vol. 29, no. 2, pp. 293-303, 2008. View at: Publisher Site | PubMed
