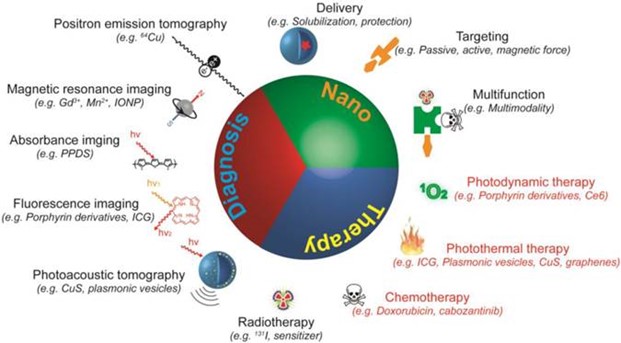
Theranostics is an emerging field of medicine which combines "therapeutics" and "diagnostics." The idea is to combine drugs and/or techniques to diagnose and treat medical conditions and monitor the response of the patient (shown in the image above) simultaneously or sequentially. This saves time and money and can also bypass some of the undesirable biological effects that may arise when these strategies are employed separately. Today, theranostics applications increasingly use nanoparticles (NPs) that unite diagnostic molecules and drugs into a single agent. The NPs act as carriers for molecular "cargo," e.g., a drug or a radioisotope to cancer patients undergoing radiotherapy, targeting specific biological pathways in the patient's body, while avoiding damage to healthy tissues. Once at their target tissue, the NPs produce diagnostic images and/or deliver their cargo. This is the cutting-edge technology of "nanotheranostics," which has become a major focus of research, albeit with many limitations to overcome.
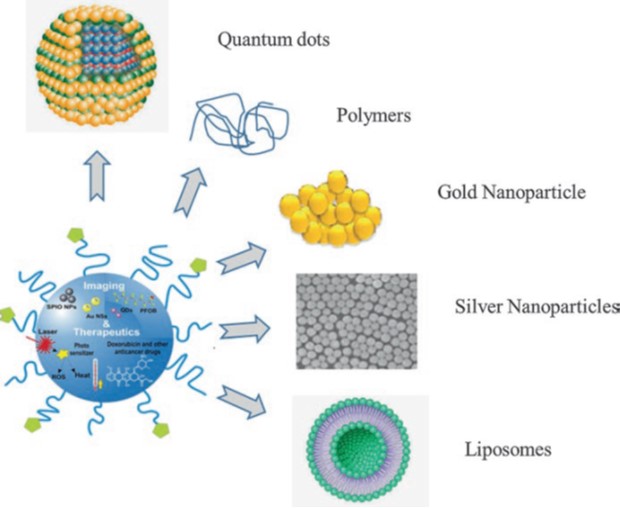
Usually most of the nanomedicines act by increasing bioavailability of the drug, protection from degradation, and controlled biodistribution in the body system. Nanotheranostics thus encompass all those nano stages that can be used for simultaneous detection and treatment of disease by providing better penetration of drugs within the body systems with reduced risks as compared to other conventional therapies. Theranostics offer new and emerging applications of nanotechnology. Nonetheless, the nanocarrier should have the capacity to accommodate multiple agents such as stabilizer, therapeutics, and targeting and imaging moieties. Several nanotheranostics platforms have been presented over the past decade. Nonetheless, most frequently used are the traditional ones (image below), namely metal (gold and silver) and silica nanoparticles (Si NPs), liposomes, quantum dots, and composite NPs.
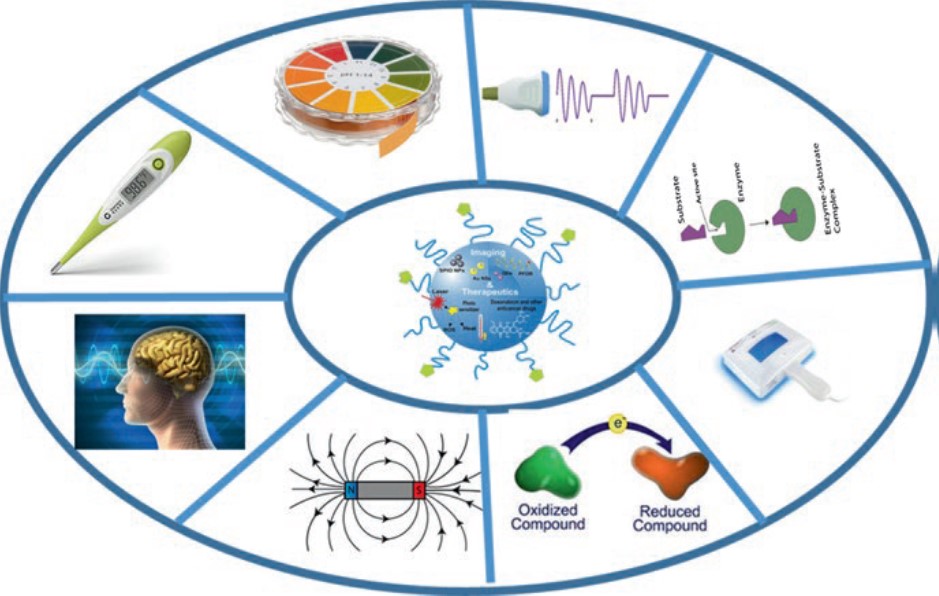
Besides offering a promising approach for the treatment of various diseases like cancer, neurological disorders, cardiovascular disorders and autoimmune disorders, nanotheranostics can be applied to solve a major public health problem of our time, antimicrobial resistance, developed by microbes causing common infections such as bacterial pneumonia, postoperative infections, and higher mortality infections including malaria, HIV, and tuberculosis that are becoming increasingly difficult to treat due to the increase in resistance. Stimulus for nanotheranostics might be external or internal (illustrated in the image below).
Concerning nanotheranostic applications in liquid tumors, research is still in its infancy compared to solid tumors. Most of the approaches done thus far are directed to the diagnosis and treatment of blood cancers, such as lymphoma and leukemia. It is surprising to note that although the physicochemical scenario of blood cancers is different than solid tumors, the same types of nanomaterials are being used. This can be clearly observed in the use of metal NPs for thermal ablation in both solid and liquid tumors. In solid tumors, the nanomaterial is anchored to the surface of the tumor, leading to an effective and non-invasive ablation, whereas in liquid tumors, the target element is in constant circulation and can lead to ablation of adjacent tissue. The same phenomenon is observed with the use of polymeric and lipid NPs as drug delivery systems in both solid and liquid tumors, where the different requirements are not clear in terms of blood-brain barrier penetration, stability, and renal clearance. This question needs to be solved and more work should be carried out to push nanotheranostics for in vivo applications in circulating tumors.
For neurodegenerative diseases, all the nanotheranostic strategies have been applied for the diagnosis and treatment of AD, and interestingly the selection of the nanomaterial is based on the therapeutic strategy (inhibition of Aβ aggregation and Cu2+ ions chelation). In both cases, fluorescence imaging modality is selected as a cost-effective and simple alternative to MRI and PET. Moreover, both therapy strategies have proven to be effective in in vivo therapy of AD, although it would be important to develop them also for the other neurodegenerative diseases.
The NPs used for the theranostics of autoimmune diseases can be colloidal-based or organic-based. The nanomaterials are mostly designed for targeting of the scavenger receptor present on the activated macrophages. The NPs permit prolonged circulation in the bloodstream and subsequent controlled release of the therapeutic drugs at the inflammation sites. Safety concerns (biodistribution in nontargeted tissues, potential inflammation, biodegradability, biocompatibility, and clearance) are the biggest obstacle that hampers the clinical application of most of the nanomedicine of RA.
For cardiovascular diseases, the research is mainly focused on the non-invasive imaging modalities such as MRI and NIR fluorescence for detection of atherosclerotic plaque. The pursuit of therapeutic agents for atherosclerosis and its potential impact remains an open field for research. Currently, there is less information about the dose, pharmacokinetics, long-term side effects, and toxicology of the NPs employed in most of the studies. Future research should also be emphasized on more detailed biocompatibility (such as blood-half-life) and immunogenicity evaluation of the NPs as well as development toward precision drug delivery and early detection of atherosclerotic plaque.
Developing smart nanotheranostics that act on the bioresponsive systems have recently evolved and offering promising result with high accuracy and efficiency at targeted point via on-demand drug release. It can be said that nanotheranostics is a promising and emerging field that can offer rapid detection and targeted delivery system that is rapid and cost-effective with reduced risks. Considering the limitation of external stimuli, numerous internal stimuli such as glucose concentrations, pH differences, redox reactions, and other ions/small biomolecules are more efficient for the designing of smart drug delivery systems. Also, the performance of nanotheranostics should be observed not only before or after but also throughout the therapy and after gaining extensive information about their genotoxicity, cytotoxicity, immunotoxicity, and cost-effectiveness, the nanotheranostics can be introduced into routine healthcare as an important element of personalized and predictive medicine.