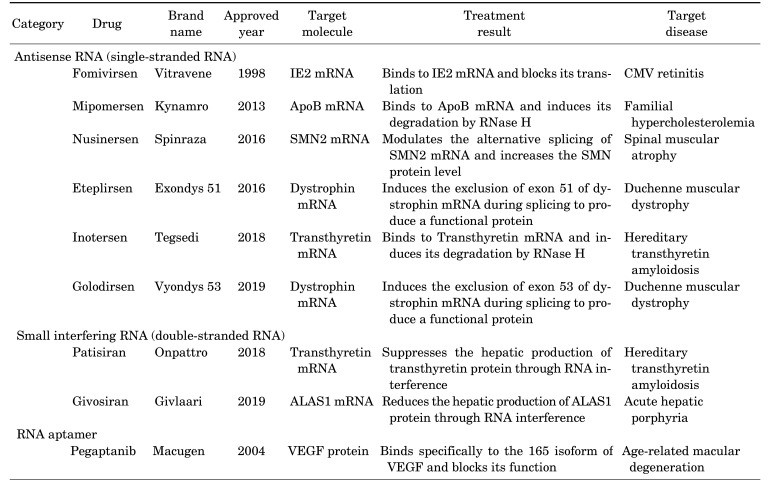
With a handful of RNA therapies already approved (figure below) and a plethora in clinical trials, patients with many previously untreatable conditions have cause for optimism. Currently, there are 7,000 recognized rare diseases, and hundreds of them should be treatable with RNA interference therapeutics. Everything is out there to be done, and everything seems possible!
For many years pharmaceutical companies have relied on small-molecule therapeutics to generate drugs. While small-molecule drugs possess certain favorable characteristics (such as ease of production, possibility of oral administration, favorable pharmacokinetics, and the ability to pass through the cell membrane), their potential is limited as they rely on the druggability of the target. Whether a biological target is druggable or not depends on a variety of factors such as the presence of suitable pockets in the protein structure in which small molecules can dock, a suitable size to accommodate binding, and the degree of polarity. Docking into a deep cavity is crucial in achieving sufficiently high binding affinities. It is estimated that out of the ~20 000 human proteins, only ~3000 are druggable. To tap into this unexplored potential, we need to look beyond small-molecule drugs. Over the years, more complex, biological macromolecules such as monoclonal antibodies (mAbs) have entered the pharmaceutical arena. Major benefits of their use, compared with small-molecule drugs, include long half-life, ability to target a broader group of proteins (due to the vast mAb repertoire), ability to be engineered to widen their applicability and increase their specificity, and lower toxicity. Disadvantages of the more complex biological macromolecules include more complicated pharmacological profiles, higher cost of production, and limits in route of administration (mostly intravenous).
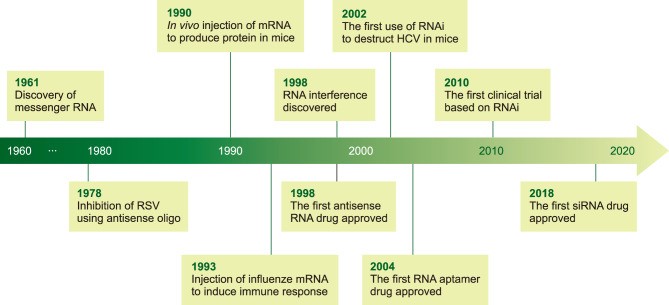
While small molecules still dominate the pharmaceutical market, biologics have started to gain a higher share in the last few years. In line with the trend of developing more specific and efficacious medicines, a new therapeutic avenue is gaining momentum – that of nucleic-acid-based therapy. Examples of such therapeutic agents include the use of oligos, plasmid DNA, mRNA, ribozymes and RNAi-related nucleic acids such as miRNA, siRNA, and short hairpin RNA (shRNA). While the use of mAbs are limited to cell surface receptors or secreted proteins, nucleic acids can interfere with protein expression itself and therefore circumvent the druggability issue during drug development. While clinical development of RNA therapeutics has faced decades of significant challenges in terms of potency and immunogenicity, in recent years, the field has gained some momentum with the recent approval of two siRNA-based drugs patisiran and givosiran within a short period of time. This, combined with a well-filled clinical pipeline of mRNA therapeutics, shows the potential of clinical development of RNA therapeutics in the coming years. RNA therapy is a safer alternative to DNA therapy and is versatile as it can either increase or decrease gene expression in order to introduce new transcripts for protein replacement therapy and more. The figure below enlists the major discoveries that led to the establishment of the RNA therapy field.
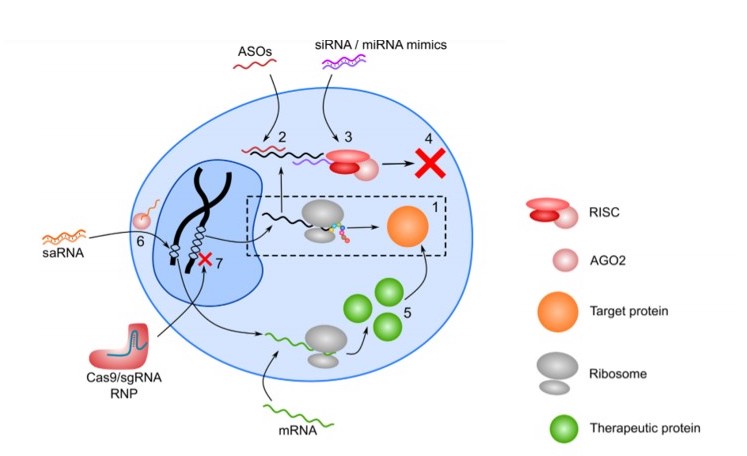
RNA therapeutics span from antisense oligonucleotides (ASOs), siRNA, miRNA, mRNA, RNA aptamers, short activating RNA (saRNA), to single guide RNA (sgRNA) for CRISPR/Cas9 systems (illustrated in the figure below). While the field has seen significant progress, some of the major obstacles in RNA therapeutics are the unstable nature (due to the high stability and activity of RNases) and high immunogenicity of the RNA molecules. Both single-stranded and double-stranded RNA molecules induce the production of type I interferons and various other proinflammatory cytokines through multiple signaling pathways. This necessitates chemical modifications of the RNA molecule to make advancement to the clinic more realistic. Such modifications can involve alterations of the ribose group, the phosphate backbone, the RNA termini, or modification of the nucleobases themselves.
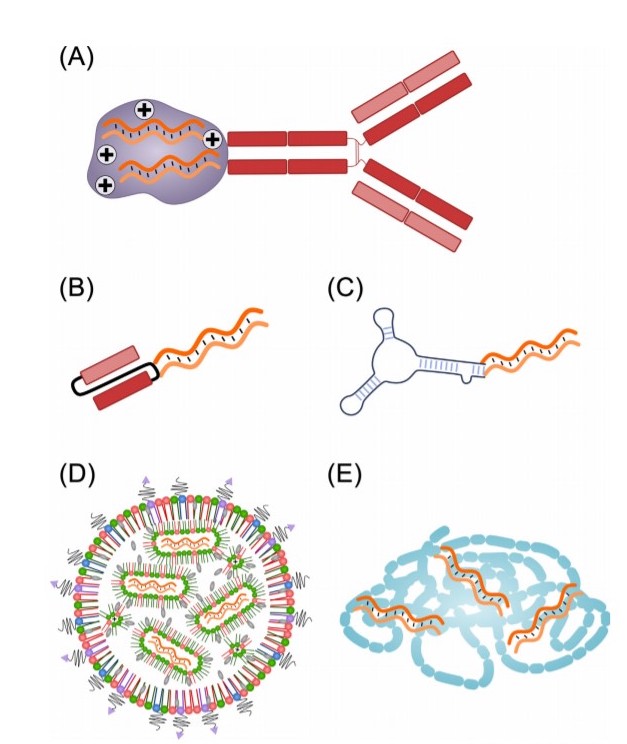
The major challenge in RNA therapeutics is delivery and difficulties in intracellular uptake of RNA molecules due to the large molecular weight and negatively charged phosphate backbone that hinders internalization. However, various carriers for the in vivo delivery of RNA molecules have been invented as depicted in the figure below: (A) antibody-RNA conjugates, (B) conjugate of RNA with a single-chain variable fragment (scFv), (C) aptamer-RNA conjugates, (D) lipid nanoparticles, and (E) cationic polymers can encapsulate RNA therapeutics by electrostatic interactions. Delivery to extrahepatic tissues might require targeting moieties.
In conclusion, the field of RNA therapeutics has seen major developments at multiple levels (targeting, RNA modifications, delivery vehicles, etc.) and a wide variety of different RNA molecules are currently at different stages of (pre-)clinical development. Owing to the invested efforts, RNA therapeutics moved from unrealistic dreams to genuine realities and many actors in the field are determined to drive the RNA revolution to the next level.