Received: Wed 15, Jul 2020
Accepted: Thu 30, Jul 2020
Abstract
The presence of tumor thrombus extending into the inferior vena cava (IVC) is a rare complication of hepatocellular carcinoma (HCC), presenting in only 3.8% of patients, resulting in a median survival of 1.9 months. Even rarer and likely with worse outcomes is a tumor extending from the IVC into the right atrium and ventricle. Here we present the case of a 55-year-old male with advanced liver cirrhosis from non-alcoholic steatohepatitis (NASH) with a diagnosis of HCC and IVC tumor thrombus invading into the right atrium and ventricle. The patient was treated with stereotactic body radiotherapy (SBRT) to 30Gy in 5 fractions delivered over 5 days targeting the right atrium, right ventricle and the outflow tract. Initially, the patient had a radiographic and clinical response to treatment, with minimal acute toxicities. Unfortunately, due to declining liver function, the patient subsequently entered hospice care and died 4 months post-treatment. This case demonstrates the poor prognosis of this advanced presentation of disease but suggests that SBRT is a safe and reasonable treatment approach for the reduction of IVC and cardiac tumor thrombus in HCC.
1. Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer death in the world [1]. Risk factors include cirrhosis as a result of alcohol, viral hepatitis, metabolic associated fatty liver disease (MAFLD), hemochromatosis, and autoimmune hepatitis. The treatment process is determined based on the patient’s functional status, liver function, tumor size, quantity, and location of the lesions.
HCC has a propensity to invade the liver vasculature and also form tumor thrombi in the portal and hepatic veins. Invasion or thrombus of a major branch of the portal vein is relatively common and is reported to occur in 10-60% of patients [1-3]. However, HCC complicated by an inferior vena cava tumor thrombus (IVCTT) is much rarer occurring in only 3.8% of patients [1]. The more advanced the thrombus, the more challenging the treatment as there is often co-existing bulky tumor volume, aggressive histology, worse baseline liver function and higher serum AFP [4]. Given these factors, the prognosis of these patients is dismal with a median survival of 1.9 months [5].
Intrahepatic tumor thrombus prohibits treatment with liver transplant or curative resection, and IVCTT adds additional treatment challenges due to the risk of pulmonary embolus and damage to the heart. However, several groups have shown that transarterial chemoembolization (TACE) is effective and safe, albeit in patients with hepatitis B and favorable liver function (Child-Pugh class A disease) [6, 7]. One report of 11 patients with hepatitis B and IVC or right atrial tumor thrombus used a combination of TACE and stereotactic body radiotherapy (SBRT) and showed a median survival of 21 months [7].
For many years, radiotherapy was not used in the management of HCC due to concerns for radiation-induced liver disease. However, given improved imaging techniques, better patient immobilization, and more conformal delivery methods, the rates of local control are high and rates of toxicity relatively low. Thus, SBRT is gaining traction as a useful tool in the management of intrahepatic HCC [8, 9]. SBRT has been successfully employed for tumors with PV, IVC and even right atrial tumor thrombus [7, 10, 11]. Again, these experiences were all in hepatitis B endemic regions but showed that SBRT was safe and had some efficacy even in tumor extending into the heart.
Based on the excellent results with SBRT for primary HCC and the promising findings with focal radiotherapy for portal vein tumor thrombosis (PVTT) and even IVC and right atrial tumor thrombus, it is reasonable that SBRT could be used for extensive clot within the heart from HCC. Here we describe the case of a patient who presented with tumor thrombus extending from his liver dome into the IVC, right atrium and ventricle who received primary SBRT.
2. Case Report
A 55-year-old male with a history of NASH, type 2 diabetes, coronary artery disease and obesity presented with abdominal pain and distension. He had an ultrasound, which showed abdominal ascites. He later had a CT at an outside hospital showing a 4.7 x 4cm lesion in the hepatic dome with a 3.0 x 4.3cm soft tissue mass extending along the suprahepatic IVC to the right atrium (Figures 1a & 1c). A small filling defect was also identified in the right ventricle, measuring 0.6cm in diameter due to a pulmonary embolus. He had an ultra-sound guided biopsy of the tumor, and it was consistent with moderately differentiated HCC. Of note, during this workup he was admitted with an acute kidney injury. At that time, his kidney lab tests demonstrated: creatinine 1.58 (nl 0.6-1.4 mg/dL), BUN 74 (nl 8-24 mg/dL) and potassium 5.6 (nl 3.5-5.0 mmol/L).
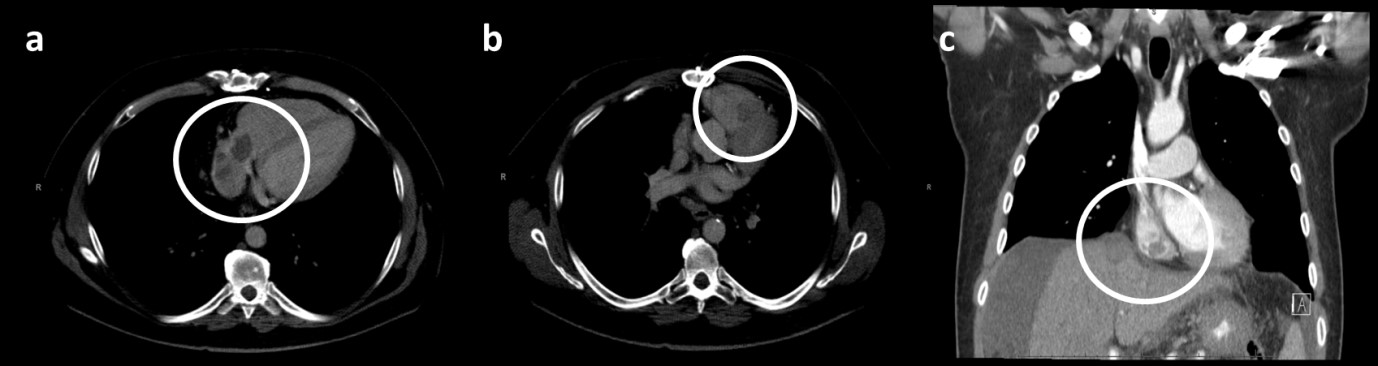
The patient’s case was discussed at the multidisciplinary tumor board and treatment options discussed were intra-arterial therapy with SIRT Y90 administration, external beam radiation therapy and/or chemotherapy. Initially, the patient was set up for SIRT; however, tracer mapping for the Y90 treatment showed increased shunting into the lungs, which is a contraindication for this treatment modality. Therefore, he was referred to radiation oncology. Prior to starting radiation therapy, the patient had a repeat chest CT (4 weeks after the initial CT), which showed the tumor thrombus within the IVC, the right atrium, and now also the right ventricle, with the invovlement of the tricuspid valve (Figure 1b). The patient’s liver function tests prior to therapy were: ALT 44 U/L (nl 7-55 U/L), AST 59 U/L (nl 8-48 U/L), total bilirubin 0.6 (nl 0.1-1.2 mg/dL), albumin 3.2 (nl 3.5-5.0 g/dL) and an international normalized ratio (INR) of 1.2 (nl <1.1). The tumor markers showed an alpha-fetoprotein (AFP) of greater than 15,000 (normal <10ng/mL) (Table 1). The patient had a paracentesis with 6400 mL of ascitic fluid drained prior to any therapy. Given his diuretic-refractory ascites and low albumin, he was classified with Child Pugh B8 disease initially.
TABLE 1: Patient’s liver and kidney lab values before and after SBRT treatment.
|
2/3 |
4/7 |
4/28 |
|
|
Pre-therapy |
2 Months Post-Therapy |
3 Months Post-Therapy |
|
|
ALT (U/L) |
44 |
70 |
71 |
|
AST (U/L) |
59 |
124 |
90 |
|
Total Bilirubin (mg/dL) |
0.6 |
6.8 |
4.2 |
|
Albumin (g/dL) |
3.2 |
3.1 |
3.1 |
|
INR |
1.2 |
2.0 |
1.1 |
|
AFP (ng/mL) |
15000+ |
1238 |
3580 |
|
Creatinine (mg/dL) |
1.58 |
0.96 |
2.12 |
|
Child-Pugh Class |
B8 |
C10 |
C10 |
ALT: Alanine Transaminase, AST: Aspartate Aminotransferase, INR: International Normalized Ratio, AFP: Alpha-Fetoprotein
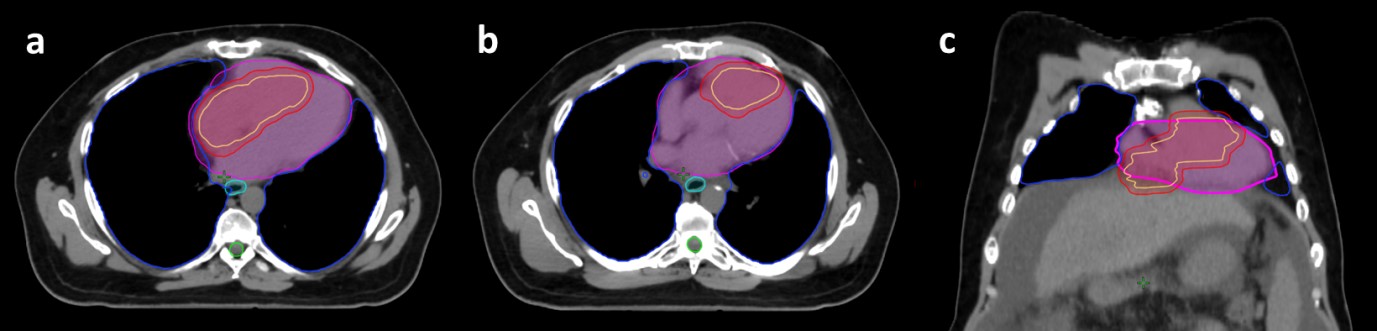
The patient underwent a CT simulation with a 4D CT. Custom immobilization was used. He was not given any IV contrast given his impaired renal function. His diagnostic images were co-registered to the CT simulation scan for treatment planning and the enhancing tumors in the IVC, right atrium and ventricle were contoured, as well as normal structures (Figure 2). The initial goal was to try to give a dose of 50Gy in 5 fractions; however, the dose was decreased to 30Gy in 5 fractions to meet the heart dose constraints (maximum dose less than 38Gy and V32 less than 15Gy). He was treated with a volumetric modulated arc plan using two 180o arcs. Radiation was given over the course of 5 consecutive days (Figure 3). The patient did well with SBRT during the treatment and required no breaks in the program. Within a week following completion of radiotherapy, he began Lenvatinib treatment at 12mg/day.
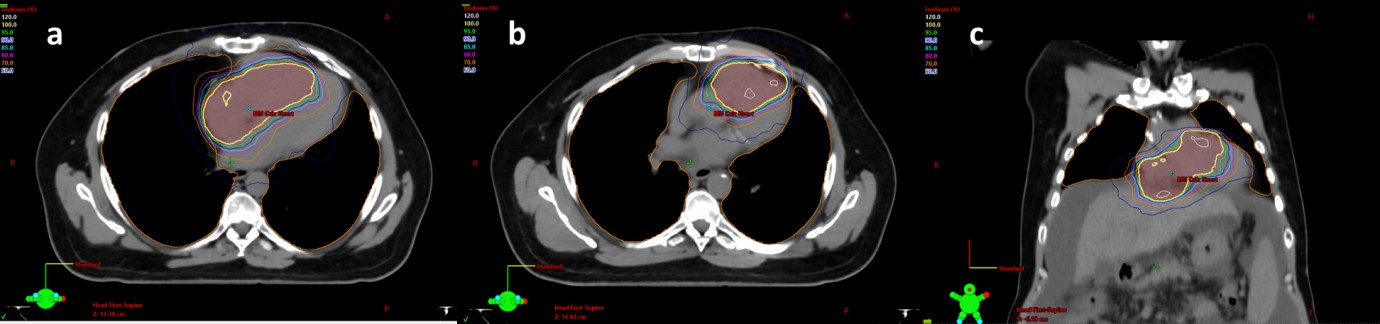
Four days later, he was admitted for dysphagia, and endoscopy revealed esophagitis secondary to radiation. He was treated conservatively with a proton pump inhibitor and discharged within 48 hours. Approximately two weeks later, the patient came into the hospital for nausea and vomiting, which was felt to be related to the radiotherapy vs. Lenvatinib, and he was again treated conservatively and discharged after an overnight stay with ondansetron. The patient was admitted one week afterward for poor renal function, and a bleeding mouth ulcer secondary to Lenvatinib treatment, and this treatment was discontinued.
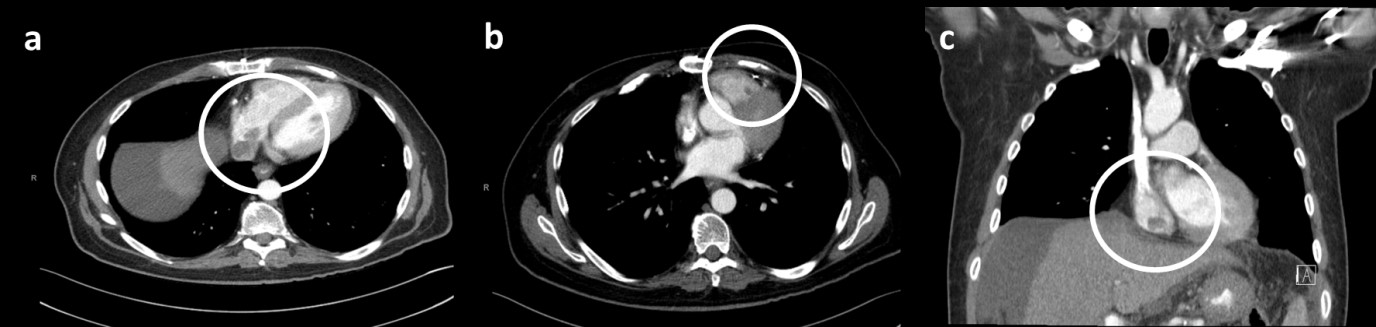
A follow-up CT imaging obtained 2 months after the completion of radiotherapy showed an interval decrease in size of the tumors extending from the IVC to the right atrium and ventricle (Figure 4). Laboratory studies showed that the AFP had correspondingly decreased from 15,000 ng/mL to 1,200 ng/mL. Unfortunately, however, the patient had a significant decline in his liver function with a dramatic rise in his bilirubin, making him a Child-Pugh class C10 (Table 1).
One month later, he was admitted to the hospital with a lower GI bleed and renal failure. His renal failure was so severe that he required in-patient dialysis. His AFP was found to be rising and was last measured at 3580 ng/mL. Unfortunately, with his underlying decompensated NASH cirrhosis and now hepatorenal syndrome, there were no other good treatment options to offer. The patient enrolled in hospice and died from progressive liver failure 4 months after initial treatment with SBRT.
3. Discussion
Here we report the first case of using SBRT to treat tumor thrombus from HCC extending from the IVC into the right atrium and ventricle. This is a very rare situation with limited treatment options and a poor prognosis. The poor prognosis can be related to other tumor factors (multifocality, grade, size, etc.), but also to portal hypertension and thromboembolic complications.
Unlike in prior reported experiences, this case was also complicated by advanced cirrhosis at the time of diagnosis. Patients with Child-Pugh C and advanced B disease at diagnosis are a competing risk of dying from their underlying liver disease. However, recent reports have shown that SBRT can be safely given for small intrahepatic HCC lesions with Child-Pugh B and C disease [12, 13]. In a recent study from the University of Massachusetts of 23 patients with Child-Pugh B and C cirrhosis, SBRT with a median dose of 40Gy in 5 fractions provided 12-month local control of 92.3% and median survival of 14.4 months, even in patients with C10 disease [13]. Unfortunately, the patient described in this case report had both advanced cirrhosis and advanced liver cancer at the time of diagnosis, thus reflecting his poor overall outcome, despite initial response to SBRT.
One of the most feared complications of treating a tumor thrombus in not only the IVC but the right atrium and ventricle is causing pulmonary thromboembolism. In the prior series of patients with IVCTT as well as in the case report used as the basis for this case with tumor in the right atrium, there were no reports of pulmonary embolism. Similarly, the patient reported here had no disease travel into the lungs. However, he was maintained on blood thinners throughout.
In the patient we describe, AFP as well as imaging were both good indicators of treatment response and then subsequent disease progression. AFP is a glycoprotein that is expressed and able to be detected in the serum in about 70% of patients with HCC. High levels of AFP, such as in the patient described, predict for high burden of disease, aggressive tumor biology, and risk of early metastatic disease. After liver-directed therapy, AFP response has been associated with overall survival and recurrence-free survival and is a useful prognostic marker [14]. Even after SBRT, normalization of AFP within 3 months is prognostic [15]. However, in this case, even though there was a large decline in AFP, the value still far exceeded normal limits and quickly began to rise again.
Despite the poor outcome of this particular patient, SBRT for tumor thrombus into the heart is a reasonable and safe treatment approach. Our patient developed grade 2 esophagitis (he was hospitalized, but only required oral medication) as well as nausea and vomiting. The treatment initially improved his quality of life and gave him some hope.
Conflicts of Interest
None.
Funding
None.
REFERENCES
- Quirk M, Kim YH, Saab S, et al. “Management of hepatocellular carcinoma with portal vein thrombosis.” World J Gastroenterol, vol. 21, no. 12, pp. 3462-3471, 2015. View at: Publisher Site
- Pirisi M, Avellini C, Fabris C, et al. “Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study.” J Cancer Res Clin Oncol, vol. 124, no. 7, pp. 397-400, 1998. View at: Publisher Site | PubMed
- Llovet JM, Bustamante J, Castells A, et al. “Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials.” Hepatology, vol. 29, no. 1, pp. 62-67, 1999. View at: Publisher Site | PubMed
- Chan SL, Chong CCN, Chan AWH, et al. “Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016.” World J Gastroenterol, vol. 22, no. 32, pp. 7289-7300, 2016. View at: Publisher Site | PubMed
- Li AJ, Zhou W, Lin C, et al. “Surgical treatment of hepatocellular carcinoma with inferior vena cava tumor thrombus: a new classification for surgical guidance.” Hepatobiliary Pancreat Dis Int, vol. 12, no. 3, pp. 263-269, 2013. View at: Publisher Site | PubMed
- Chung SM, Yoon CJ, Lee SS, et al. “Treatment outcomes of transcatheter arterial chemoembolization for hepatocellular carcinoma that invades hepatic vein or inferior vena cava.” Cardiovasc Intervent Radiol, vol. 37, no. 6, pp. 1507-1515, 2014. View at: Publisher Site | PubMed
- Duan F, Yu W, Wang Y, et al. “Trans-arterial chemoembolization and external beam radiation therapy for treatment of hepatocellular carcinoma with a tumor thrombus in the inferior vena cava and right atrium.” Cancer Imaging, vol. 15, pp. 7, 2015. View at: Publisher Site | PubMed
- Bujold A, Massey CA, Kim JJ, et al. “Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma.” J Clin Oncol, vol. 31, no. 13, pp. 1631-1639, 2013. View at: Publisher Site | PubMed
- Wahl DR, Stenmark MH, Tao Y, et al. “Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma.” J Clin Oncol, vol. 34, no. 5, pp. 452-459, 2016. View at: Publisher Site | PubMed
- Im JH, Yoon SM, Park HC, et al. “Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area.” Liver Int, vol. 37, no. 1, pp. 90-100, 2017. View at: Publisher Site | PubMed
- Shui Y, Zhu X, Wu J, et al. “Stereotactic body radiotherapy as the initial treatment for hepatocellular carcinoma with extensive inferior vena cava and atrium tumor thrombus.” Onco Targets Ther, vol. 12, pp. 5299-5303, 2019. View at: Publisher Site | PubMed
- Culleton S, Jiang H, Haddad CR, et al. “Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma.” Radiother Oncol, vol. 111, no. 3, pp. 412-417, 2014. View at: Publisher Site | PubMed
- Lee P, Ma Y, Zacharias I, et al. “Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma in Patients With Child-Pugh B or C Cirrhosis.” Advances in Radiation Oncology, 2020. View at: Publisher Site
- He C, Peng W, Liu X, et al. “Post-treatment alpha-fetoprotein response predicts prognosis of patients with hepatocellular carcinoma A meta-analysis.” Medicine (Baltimore), vol. 98, no. 31, pp. e16557, 2019. View at: Publisher Site | PubMed
- Jung J, Yoon SM, Han S, et al. “Alpha-fetoprotein normalization as a prognostic surrogate in small hepatocellular carcinoma after stereotactic body radiotherapy: a propensity score matching analysis.” BMC Cancer, vol. 15, pp. 987, 2015. View at: Publisher Site | PubMed
