Received: Tue 18, Aug 2020
Accepted: Mon 31, Aug 2020
Abstract
This is an observational study of objective responses of Glioma patients to Pharmaceutical Grade Synthetic Cannabidiol. 14 assessable cases were identified, 9 showed a clinical response, 5 showed no effect.
1. Introduction
The use of a whole variety of cannabis oils of questionable quality, none of which were pharmaceutical grade, and all bought on the internet has been a matter of routine by cancer patients. No anticancer effect of these oils has been noted [1-3]. Currently, it is illegal to buy cannabis oil on the internet as the Medicines and Health Regulatory Agency has defined CBD as a medicinal product, which can only be prescribed under the Pharmaceutical Specials scheme, as it is not currently a licensed medicinal product [4]. Cannabidiol targets CB1 and CB2 receptors [2-4], which have increased expression in gliomas, generally speaking, CB1 and CB2 receptors are upregulated in tumor tissue.
The phytocannabinoids are a group of chemicals extracted from the cannabis plant. A number of them are able to impede cancer cell growth, induce apoptosis and autophagy, and inhibit angiogenesis. The most widely known phytocannabinoid is Δ9-tetrahydrocannabinol (THC), and although it possesses these anticancer effects, it is also psychoactive, which has arguably hampered its clinical development. It is thought that these actions are mediated, in part, by binding to cannabinoid receptors that are expressed on a number of tissue types [8]. As one type of the receptor is found exclusively on brain cells, studies using THC have focused on this tissue type. In vitro data were promising, and in 2016, a pilot clinical study in patients with glioblastoma multiforme indicated THC was safe; however, no clear activity was reported [9]. The dosages were possibly on the conservative side, to minimize psychoactivity that would naturally restrict the use of THC as a drug.
Of the 80+ phytocannabinoids, THC is possibly the only one to exhibit this psychoactivity. More recently, studies have diverted away from THC and focused on other cannabinoids. The next most abundant compound is cannabidiol (CBD), which has a low affinity for the canonical cannabinoid receptors. In contrast to THC, in its pure state, according to the World Health Organization, CBD did not have abuse potential and caused no harm [10]. Studies have shown that in addition to being able to induce cell death directly, it is also capable of interfering with intracellular signalling [11]. Alterations to pathways such as the PI3K/AKT/mTOR and the ERK, suggests that CBD can modify the way certain cancer cells react to other treatments. Indeed, studies have shown that combining CBD with conventional chemotherapy such as cytarabine and vincristine can lead to enhanced anticancer activity through modifications to these signalling pathways [12, 13]. Furthermore, the sequence in which these drugs are administered can also influence overall activity. Studies have also indicated that in certain leukaemia cell lines, CBD can increase the expression of the cyclin-dependent kinase inhibitor p21 [13]. This increased level appears to be maintained by CBD, which inadvertently impedes cell death. Cytotoxicity could be restored in these cells if the treatment regimen was altered to allow for a temporary cessation of exposure to CBD. Thus, the general efficacy of CBD may also be altered by adapting treatment protocols that include “drug-free” phases [13].
Brain tumors are on the rise. The brain tumor incidence rate has increased by 36% since the early 1990s [14]. The findings of a number of studies designed to examine the role of cannabinoids in the management of cancer symptoms varied [15]. The most recent prospective analysis of nearly 3,000 patients using medical marijuana showed that a large proportion of patients reported improvement in their condition [16]. Patients often feel that conventional therapies are not working for them, and so they search the internet for alternative medicines. It is here that they find stories about cannabis working in patients with cancer, and understandably feel it is a route for them. The cannabis products they use vary and can be in the form of whole-plant extracts or purified oils; however, whatever the source, they self-prescribe dosages. A number of anecdotal positive responses have been reported, which sustains the interest in this type of medication. We have previously reported on objective clinical responses in a variety of cancer patients using pharmaceutical grade synthetic cannabidiol (PGSC) [17].
A recent observational study treating nine patients with Glioblastoma Multiforme (GBM) produced an outcome in that one patient died, and the other eight had a median survival of 22.3 months (range=7-47 months). This is longer than would have been expected [18]. Over five years ago, we decided to assess the potential use of PGSC in glioma patients.
2. Materials and Methods
Patients were given synthetic PGSC (STI Pharmaceuticals), under the Pharmaceutical Specials scheme in oily drops at 5% (w/v) in 20ml bottles. Each drop contains 1mg of synthetic CBD in neutral oil. This was prescribed on an informed consent basis. Of the 12 patients described here in this observational study, every patient in this study signed an informed consent allowing anonymous use of their data. Medicinal use of synthetic cannabinoids has been extensively reviewed in a recent paper [19].
CBD was administered on a three days on and three days off basis, which was clinically found to be more effective than giving it as a continuous dose. The average dose was 10mg twice a day. For increased tumor mass, the dose was increased, in some cases up to 30 drops (30mg). We clearly demonstrated that there is a dose-response relationship in the treatment of cancer using PGSC. In a number of cases where there was stable disease, the dose was reduced to 5 drops (5mg) twice a day.
3. Results
The results are shown in (Tables 1 & 2). Of the 14 assessable cases, 9 showed a clinical response. Five showed no effect. We were unable to define a maximum tolerated dose of CBD, as there was an absence of significant side effects. The only noted side effects were some degree of drowsiness in those patients who received a dose of 20mg twice a day or above. This side effect did not persist. The results of our glioma cohort previously treated with PGSC is reported here in significantly more detail than in our previously published study [16]. This has been in response to many requests for more details on outcomes.
TABLE 1: Outcomes – brain tumours.
|
Tumour Free |
0 |
|
Stable Disease |
1 |
|
Extended Median Survival |
8 |
|
No Effect |
5 |
|
Died |
12 |
|
CBD Only treatment |
14 |
|
Lost to follow up |
1 |
|
TOTAL CASES |
15 |
TABLE 2: Gliomas - a detailed list of patients included in this study.
|
Age |
Diagnosis |
Standard Treatments |
CBD only treatment? |
|
|
M 5
(BR1) |
Anaplastic Ependymoma |
None |
ü |
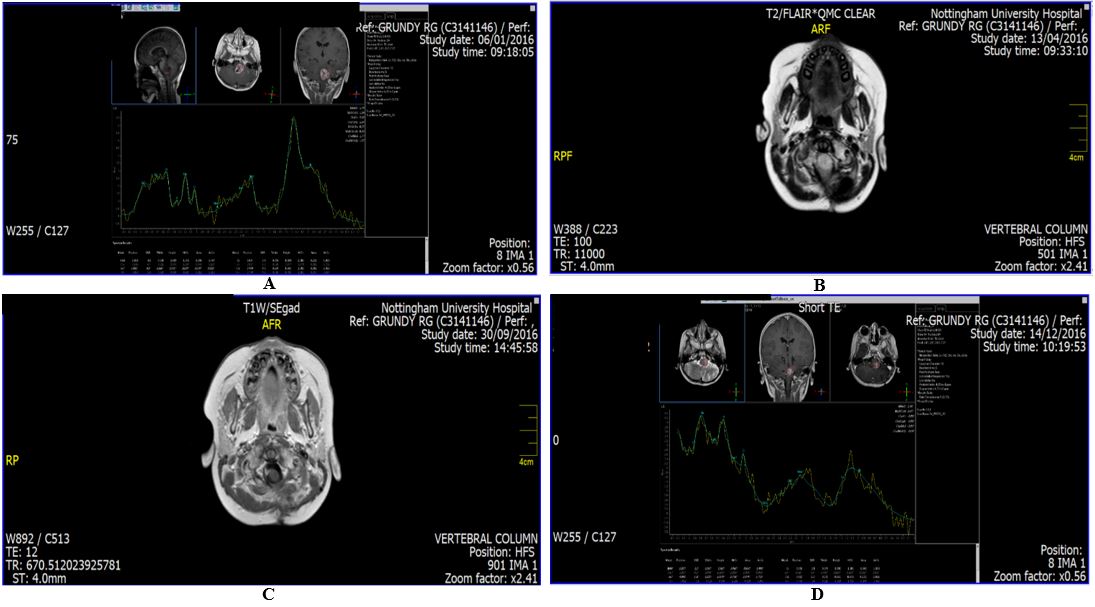
Patient first presented in February 2016 with an expected survival of three months. He had failed all standard treatments, surgery on two occasions, followed by Chemotherapy and Conformal Photon Radiotherapy. No further treatment options were available. A scan carried out in December 2016 showed that the tumour had decreased by 60% following the use of PGSC. Subsequent scans continued to show stable disease. PGSC was the only treatment. Four scans with the scan report at the top of each scan are appended. (See: Figure 1 A-D). |
|
M 59
(BR2) |
Tanycitic Ependymoma Grade 2 |
None |
ü |
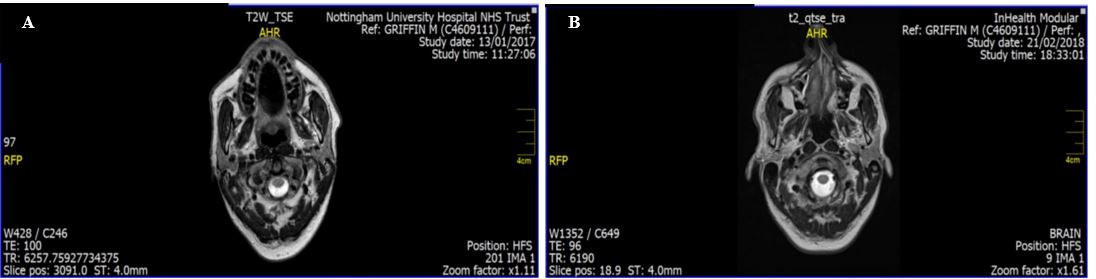
Diagnosed in June 2013. He had all standard treatments apart from Chemotherapy, which he refused. We started him on PGSC in July 2016 at which time his expected survival was three months. Scan taken in January 2017 showed tumour reduction, at which stage he switched from PGSC to Cannabis Oil bought on the Internet. Further scans carried out in February 2018 showed doubling of tumour size. He has since restarted PGSC. The scans are appended. (See: Figure 2 A & B). |
|
F 10 (BR3) |
Anaplastic Astrocytoma |
|
ü |
This patient had an expected survival of three months when we saw her at the end of November 2011. PGSC was the only treatment, this patient died in February 2012, so there was no increase in expected survival. |
|
M 7 (BR4) |
Anaplastic Ependymoma |
|
ü |
All standard treatments had failed. We saw him at the end of June 2017 and his expected survival at that time was three months. The patient died in July 2018, PGSC was the only treatment, so we extended his expected survival. |
|
M 57 (BR5) |
Glioblastoma Multiforme |
|
ü |
Diagnosed in February 2018. He had all standard treatments but refused Temozolomide. We saw him in March 2018 and started him on PGSC. His expected survival when we first saw him was two months, he died in October 2018, so he had his median survival extended with PGSC as the only treatment option. |
|
F 68 (BR6) |
Glioblastoma Multiforme |
|
ü |
We first saw this patient in May 2018 following all standard treatments. Her expected survival at the time we saw her was six months. PGSC was the only treatment, she died in August 2019, so no extended survival. |
|
M 10 (BR7)
|
Anaplastic Ependymoma |
|
ü |
We first had contact with this patient on 10th January 2018, he commenced PGSC in February 2018. His expected survival at the time we first saw him was three months. He died in July 2018, so there was some short increase in expected survival. |
|
F 14 (BR8) |
Anaplastic Ependymoma |
|
ü |
We first saw this patient in April 2017 with Anaplastic Ependymoma, failure of standard treatments and clear progressive disease. She had an expected survival of six months when we saw her, she died in January 2018, so there was no increase in expected survival. |
|
M 9 (BR9) |
Diffuse Interstitial Pontine Glioma |
|
ü |
We first saw this patient in April 2017 with Diffuse Interstitial Pontine Glioma. This followed all standard treatments. His expected survival when we saw him was three months. He has been lost to follow up. |
|
M 69 (BR10) |
Left Occipital Glioblastoma Multiforme |
|
ü |
We first saw this patient in July 2014 with a Left Occipital Glioblastoma Multiforme. He was post all standard treatments and his expected survival when we saw him was one year. He died one year later, so there was no effect from the PGSC, which was the only treatment. |
|
M 47 (BR11) |
Recurrent Right Occipital Lobe Glioma
|
|
ü |
We first saw this patient in December 2015 with a Recurrent Right Occipital Lobe Glioma. Expected survival was six months. We put him on PGSC. He died in December 2016, so he had extended survival. |
|
M 42 (BR12) |
Recurrent Glioblastoma Multiforme |
|
ü |
We saw this patient in November 2015 with a Recurrent Glioblastoma Multiforme. He had failed all standard treatments. Expected survival was six months when we first saw him. We were last in touch with him in December 2016 and he was still clinically well, with PGSC as the only treatment. So, he had extended survival. |
|
F 6 (BR13) |
Anaplastic Ependymoma |
|
ü |
We saw this patient in May 2018 with Anaplastic Ependymoma. All standard treatments had failed. She had an expected survival at the time we saw her of three months. She died in January 2019, so she extended her expected survival with PGSC as the only treatment. |
|
M 56 (BR15) |
Glioblastoma Multiforme |
|
ü |
We saw this patient in April 2017 with Glioblastoma Multiforme. At that time his expected survival was three months. PGSC was the only treatment. He died in July 2017, so no significant extension of expected survival. |
|
F 9 (BR16) |
Diffuse Interstitial Pontine Glioma |
|
ü |
We saw this patient in October 2015 Diffuse Interstitial Pontine Glioma, following all standard treatments. Expected survival at that time was six months. PGSC was the only treatment. She died in January 2018, so there was significant extension of expected survival. |
4. Discussion
PGSC is shown in this paper to have significant anticancer effects. This study is an observational study, and prospective randomized control studies are worth doing using this approach, as it is free from side effects. The weakness of this study is that it is an observational study.
5. Conclusion
PGSC has an objective anticancer effect in brain tumor patients. To elucidate this further, then further studies addressing the weakness of this particular study are worth carrying out.
Conflicts of Interest
None.
Funding
This study was supported by a research grant from Alinova Biosciences.
Acknowledgements
The writing of this study has been made possible by a grant from Alinova Biosciences. Julian Kenyon would like to acknowledge the contributions made by Andrew Davies and Colin Stott to this paper.
REFERENCES
- Radmila Pavlovic, Giorgio Nenna , Lorenzo Calvi, et al. “Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations.” Molecules, vol. 23, no. 5, pp. 1230, 2018. View at: Publisher Site | PubMed
- Marcel O Bonn Miller, Mallory J E Loflin, Brian F Thomas, et al. “Labeling Accuracy of Cannabidiol Extracts Sold Online.” JAMA, vol. 318, no. 17, pp. 1708-1709, 2017. View at: Publisher Site | PubMed
- Ryan Vandrey, Jeffrey C Raber, Mark E Raber, et al. “Cannabinoid Dose and Label Accuracy in Medical Cannabis Products.” JAMA, vol. 313, no. 24, pp. 2491-2493, 2015. View at: Publisher Site | PubMed
- MHRA “Regulatory status of products containing CBD.” 2016.
- Christopher J Fowler, Kent Olov Jonsson, Anna Andersson, et al. “Inhibition of C6 glioma cell proliferation by anandamide, 1-arachidonoylglycerol, and by a water soluble phosphate ester of anandamide: variability in response and involvement of arachidonic acid.” Biochem Pharmacol, vol. 66, no. 5, pp. 757-767, 2003. View at: Publisher Site | PubMed
- Burkhard Hinz, Robert Ramer, Karin Eichele, et al. “Up-regulation of cyclooxygenase-2 expression is involved in R(+)-methanandamide-induced apoptotic death of human neuroglioma cells.” Mol Pharmacol, vol. 66, no. 6, pp. 1643-1651, 2004. View at: Publisher Site | PubMed
- Ma C, Wu T T, Jaing P C, et al. “Anticarginogenic activity of anandamide on human glioma in vitro and in vivo.” Mol Med Rep, vol. 13, no. 2, pp. 1558-1562, 2016. View at: Publisher Site
- RG Pertwee “The pharmacology of cannabinoid receptors and their ligands: an overview.” Int J Obes (Lond), vol. 30, no. 1, pp. S13-S18, 2006. View at: Publisher Site | PubMed
- M Guzmán, M J Duarte, C Blázquez, et al. “A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme.” Br J Cancer, vol. 95, no. 2, pp. 197-203, 2006. View at: Publisher Site | PubMed
- WHO Online Q&A “Cannabidiol (compound of cannabis).” 2017.
- Paola Massi, Marta Solinas, Valentina Cinquina, et al. “Cannabidiol as potential anticancer drug.” Br J Clin Pharmacol, vol. 75, no. 2, pp. 303-312, 2013. View at: Publisher Site | PubMed
- Katherine A Scott, Angus G Dalgleish, Wai M Liu “Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration.” Int J Oncol, vol. 51, no. 1, pp. 369-377, 2017. View at: Publisher Site | PubMed
- Katherine Ann Scott, Sini Shah, Angus George Dalgleish, et al. “Enhancing the activity of cannabidiol and other cannabinoids in vitro through modifications to drug combinations and treatment schedules.” Anticancer Res, vol. 33, no. 10, pp. 4373-4380, 2013. View at: PubMed
- Cancer Research UK “Brain, other CNS and intracranial tumours incidence statistics”.
- Matthew R D Brown, W Paul Farquhar Smith “Cannabinoids and cancer pain: A new hope or a false dawn?” Eur J Intern Med, vol. 49, pp. 30-36, 2018. View at: Publisher Site | PubMed
- Lihi Bar Lev Schleider, Raphael Mechoulam, Violeta Lederman, et al. “Prospective analysis of safety and efficacy of medical cannabis in large, unselected population of patients with cancer.” Eur J Intern Med, vol. 49, pp. 37-43, 2018. View at: Publisher Site | PubMed
- Julian Kenyon, Wai Liu, Angus Dalgleish “Report of Objective Clinical Responses of Cancer Patients to Pharmaceutical-grade Synthetic Cannabidiol.” Anticancer Res, vol. 38, no. 10, pp. 5831-5835, 2018. View at: Publisher Site | PubMed
- Rudolf Likar, Markus Koestenberger, Martin Stultschnig, et al. “Concomitant Treatment of Malignant Brain Tumours With CBD - A Case Series and Review of the Literature.” Anticancer Res, vol. 39, no. 10, pp. 5797-5801, 2019. View at: Publisher Site | PubMed
- P Muralidhar Reddy, Nancy Maurya, Bharath Kumar Velmurugan “Medicinal Use of Synthetic Cannabinoids-a Mini Review.” Current Pharmacol Rep, vol. 5, pp. 1-13, 2019. View at: Publisher Site
