Received: Thu 24, Feb 2022
Accepted: Mon 21, Mar 2022
Abstract
This is a report of two cases of radioactive iodine 131- induced Graves’ disease, with a literature review on the pathogenesis and treatment of this condition. The patients initially presented with toxic nodular goiter and received radioactive iodine. A few months later, they came back with severe hyperthyroidism and elevated thyrotropin receptor antibodies. Few similar cases have been reported and attributed to an immunologic response with underlying genetic predisposition. There are no guidelines on the best way to treat this condition.
Keywords
Graves’, iodine-induced, thyroid, hyperthyroidism, thyrotropin receptor antibodies
1. Introduction
This is a report of two cases of radioactive iodine 131- induced Graves’ disease in patients who initially presented with autonomous nodular goiter.
2. Case 1
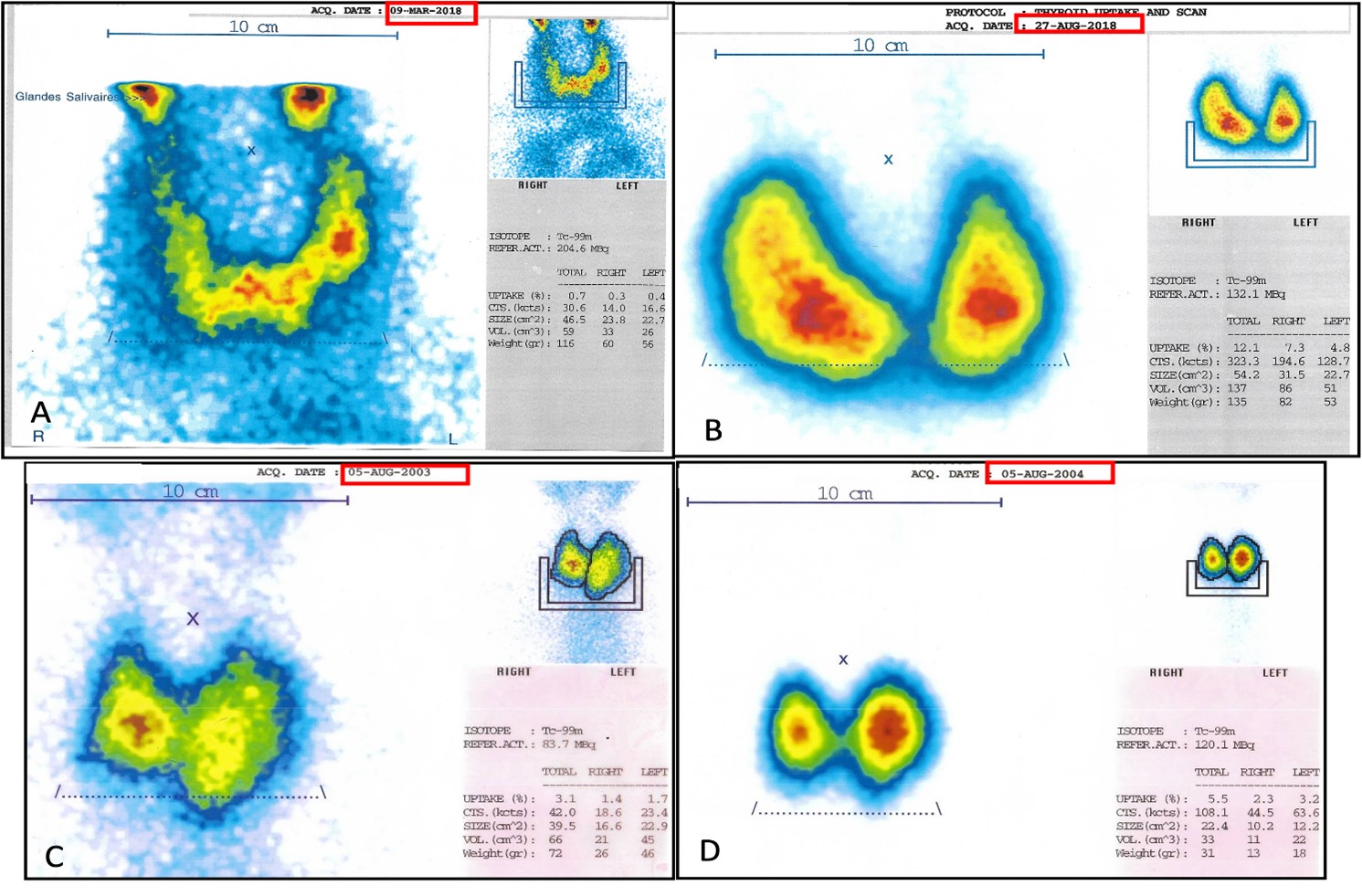
The first case is about a 78-year-old man with dyslipidemia as his sole cardiovascular risk factor. He was referred to our clinic in March 2018 with a contrast-enhanced chest CT showing a nodular goiter. A subsequent blood test (Table 1) revealed subclinical hyperthyroidism: thyroid-stimulating hormone (TSH) of 0.03 and free T4 (FT4) of 1.26. Anti-thyroid peroxidase (anti-TPO) were 154, and anti-thyroglobulin antibodies were 123. Thyroid scintigraphy showed weak uptake of 0.7% (Figure 1A) with a salt and pepper appearance. Our two differential diagnoses were thyroiditis and toxic nodular goiter with low uptake because of recent contrast injection. A follow-up thyroid blood test was ordered 2 months later: the TSH remained low at <0.06, with an FT4 of 1.12, which made the possibility of thyroiditis unlikely. A toxic nodular goiter was diagnosed, and the patient received 30 mCi of radioactive iodine 131 [1]. Three months later, a follow-up blood test revealed that hyperthyroidism had worsened with a TSH of 0.004 and an FT4 of 3.6. Twenty days later, the patient came back with clinical manifestations of thyroid storm, with an FT4 of 274 and a TSH of 0.01. The thyrotropin receptor antibodies (TRAb) were measured and came back at 100, and thyroid scintigraphy revealed an uptake of 12.1% (Figure 1B). The diagnosis of iodine-131-induced Graves’ disease was made, and the patient was prescribed carbimazole and beta-blockers. The TRAb levels then very slowly decreased to reach 2.55 in September 2020.
3. Case 2
The second case is about a 69-year-old woman with hypertension, dyslipidemia, chronic bronchitis due to cigarette smoking, a diastolic heart failure and a thyroid nodular goiter known for 30 years. She was referred to our clinic for shortness of breath, palpitations, weight loss and a cardiac ultrasound revealing concentric left ventricular hypertrophy. Subsequent testing (Table 1) showed a TSH of 0.0021 and an FT4 of 17.08, her previously known multinodular goiter on thyroid ultrasound and an uptake of 3.1% at 20 minutes on thyroid scintigraphy (Figure 1C). She received radioactive iodine-131 (30 mCi) [1]. A follow-up thyroid blood test came back normal: TSH 2.21, FT4 11.85. Six months later, she came back complaining of palpitations, fatigability, a hand tremor, nervosity, and a 10 kg weight loss. A thyroid blood test showed a TSH < 0.002 and an FT4 of 3.2, with elevated TRAb at 8.1 and positive anti-thyroid peroxidase (anti-TPO) at >1000. Thyroid scintigraphy showed an uptake of 5.5% at 20 minutes (figure 1D). A diagnosis of Graves’ disease secondary to radioactive iodine-131 was made. She was started on carbimazole, and after normalization of her FT4 (0.9 ng/dL), she received a second dose of radioactive iodine-131 (15 mCi). A follow-up blood test showed a TSH of 21.7. As a result, she was put on L-thyroxin. One year later, she came back complaining of palpitations and weight loss. Her TSH was low, and she had exophthalmia. Her TRAb level was 97.18. Thyroid replacement was stopped, and she was put back on carbimazole. A few months later, the patient’s thyroid markers and symptoms were still not controlled, and she therefore had to be put on a block and replace therapy. She has been lost to follow-up since then.
TABLE
1:
Thyroid panel of both patients at baseline and after iodine treatment.
|
Normal range |
Patient 1 |
Patient 2 |
||
|
Initial |
TSH |
0.4-4
mIU/L |
0.03 |
0.021 |
|
FT4 |
0.7-18
ng/dL |
1.26 |
17 |
|
|
Anti-thyroid
peroxidase (anti-TPO) |
0.0-9.0
IU/mL |
154 |
- |
|
|
Anti-thyroglobulin
antibodies |
<
20 IU/mL |
123 |
- |
|
|
TRAb |
<1.8
IU/mL |
- |
- |
|
|
Uptake |
0.70% |
3.10% |
||
|
After iodine treatment |
TSH |
0.004 |
<
0.002 |
|
|
FT4 |
3.6 |
3.2 |
||
|
Anti-thyroid
peroxidase (anti-TPO) |
- |
>
1000 |
||
|
Anti-thyroglobulin
antibodies |
- |
- |
||
|
TRAb |
100 |
8.1 |
||
|
Uptake |
12.10% |
5.50% |
4. Discussion
The pathogenesis of toxic adenoma and Graves’ disease are extremely different. Therefore, the pathogenesis of radioiodine-induced Graves’ disease is intriguing. There are a few other reported cases of Graves’ disease induced by radioactive iodine-131. Some patients with Graves’ disease treated with radioactive iodine-131 presented a transient rise in their TRAb and thyroglobulin levels afterward. This rise has been attributed to an immunologic response caused by the release of thyroid antigens from destroyed follicular cells [2]. This mechanism implicates an initial necrosis of thyroid cells with the release of thyroid antigens resulting in autoimmunity against the thyroid [3, 4]. Another immune mechanism that has been suggested is an increase in circulating thyroid-stimulating immunoglobulins [5]. Immune imbalance has also been hypothesized: radiodine would be responsible for killing local suppressor T cells instead of creating a new antigen [6]. None of these mechanisms has been validated.
Like our two patients, other patients had toxic nodular goiters that transformed into Graves’ disease-like hyperthyroidism after RAI (radioactive iodine) therapy [5]. This uncommon complication usually manifests 3-6 months after therapy [5, 7, 8]. It might be due to a genetic predisposition, especially HLA DR3, found in high frequencies in Graves’ disease [2, 7]. Furthermore, high anti-thyroid peroxidase (anti-TPO) levels measured before treatment with iodine-131 might also predispose to the development of high TRAb values and Graves’ disease-like hyperthyroidism [2, 9]. Indeed, it has been reported that the incidence of Graves’ disease post radioactive iodine-131 increases from 5% if anti-TPO levels are negative, to 22% in patients with positive anti-TPO levels before treatment [2, 5, 9]. Interestingly, our first patient’s anti-thyroid peroxidase antibodies (anti-TPO, N ≤ 9IU/mL) antibodies were elevated (154) before receiving his radioactive iodine-131 (RAI).
Shen et al. [10] published a case about a woman known to have a long-standing history of Hashimoto disease who developed hyperthyroidism. She was diagnosed with a toxic adenoma according to thyroid echography and scintigraphy and a normal level of TRAB. She received radioiodine-131 therapy (15 mCi) and developed Graves’ disease four months later. The patient was subsequently treated with another lower dose of radioactive iodine-131 (12 mCi), and slight hypothyroidism was observed on follow-up. She received levothyroxine substitution. This case is very similar to our second patient, who received another dose of radioactive iodine-131 after developing Graves’ disease. Unlike the case reported by Shen et al., however, our patient presented a recurrence of her Graves’ disease, which was very difficult to control, necessitating block and replace therapy with multiple changes of dosage. The other difference between these two cases is the doses of radioactive iodine-131 administered. The patient of Shen et al. received 15 mCi of radioactive iodine-131 for her toxic adenoma, and then 12 mCi for the treatment of her Graves’ disease, whereas our patient received 30 mCi for her toxic goiter and 15 mCi for her Graves’ disease.
Zgubieński et al. [11] reported the case of a 49-year-old woman with papillary thyroid microcarcinoma who underwent total thyroidectomy. Post-op immunoassays showed normal concentrations of TRAb (0.444 IU/L) and thyroxin-binding globulin (TBG) 86.91 IU/mL with abnormally high concentrations of anti-TPO 108.60IU/mL. After her surgery, the patient underwent a whole-body scan with 185 MBq of radioactive iodine-131 after administration of recombinant TSH. It showed a negligible uptake on the left side of the thyroid bed and no abnormal uptake outside the thyroid bed. However, she developed clinically significant orbitopathy three months later with elevated TRAb (40 IU/mL). She was given oral methylprednisolone therapy, and four months later, her TRAb levels decreased, and clinical improvement of her orbitopathy was observed. Methylprednisolone was eventually stopped.
Another case was described by Aoyama et al. [12] about a patient with Graves’ hyperthyroidism induced by RAI administered to treat metastatic lesions of a follicular thyroid carcinoma. The patient initially underwent a right hemithyroidectomy. Two years later, metastatic lesions to the bones and lungs were discovered. She, therefore, underwent remnant thyroid resection. At that point, the TRAb level was negative. After her second surgery, she received levothyroxine suppressive therapy and four courses of RAI therapy (100 mCi once a year for four years). Despite that, iodine-131 scintigraphy still showed intense uptake in the metastatic lesions without a decrease in their size or number. Soon after her RAI therapy, she began to experience palpitations and tremors, and blood tests showed hyperthyroidism with positive TRAb. The two mechanisms considered to elevate TRAb levels were repeated radioactive iodine-131 therapy and TSH receptors functioning as antigens of TRAb in metastatic tumors, as theorized by Yoshimura et al. [13]. She was diagnosed with Graves’ disease and was put under thiamazole, which failed to control her hyperthyroidism. Thereafter, a fifth course of RAI (100 mCi) was performed, after which an iodine-131 scintigraphy revealed massive uptake in the metastatic lesions. Finally, after stereotactic radiotherapy and RAI therapy, sizes and numbers of pulmonary metastases decreased, other metastatic lesions stabilized, and thyroid functions and TRAb were reduced. Thiamazole was gradually tapered and then stopped.
5. Conclusion
To conclude, our two cases raise some concerns in our daily practice. First, it is essential to keep in mind that the use of radioactive iodine-131 in predisposed patients could lead to severe manifestations of Graves’ disease. In fact, iodine-131 may generate antigens that stimulate TRAb that were still dormant. Second, the literature lacks guidelines concerning the treatment of iodine-131-induced Graves’ disease. Heterogeneity in response, as shown by opposite responses to a second dose of iodine-131 in our patients, creates the need for close follow-up and individualized treatment.
Consent
An informed consent was obtained from both patients for the publication of this case report.
REFERENCES
[1]
Douglas
S Ross, Henry B Burch, David S Cooper, et al. “2016 American Thyroid
Association Guidelines for Diagnosis and Management of Hyperthyroidism and
Other Causes of Thyrotoxicosis.” Thyroid, vol. 26 no. 10, pp. 1343-1421,
2016. View at: Publisher
Site | PubMed
[2]
B
Nygaard, J Faber, A Veje, et al. “Transition of nodular toxic goiter to
autoimmune hyperthyroidism triggered by 131I therapy.” Thyroid, vol. 9,
no. 5, pp. 477-481, 1999. View at: Publisher
Site | PubMed
[3]
Anis
Rehman, Silvana Obici, Abid Yaqub “Radioiodine Therapy-Induced Conversion of
Toxic Adenoma to Graves’ Disease.” Cureus, vol. 12, no. 6, pp. e8683,
2020. View at: Publisher
Site | PubMed
[4]
B
Nygaard, J H Knudsen, L Hegedüs, et al. “Thyrotropin receptor antibodies and
Graves' disease, a side-effect of 131I treatment in patients with nontoxic
goiter.” J Clin Endocrinol Metab, vol. 82, no. 9, pp. 2926-2930, 1997.
View at: Publisher
Site | PubMed
[5]
Yakup
Yürekli, Arzu Cengiz, Engin Güney “Graves Disease Induced by Radioiodine
Therapy for Toxic Nodular Goiter: A Case Report.” Mol Imaging Radionucl Ther,
vol. 24, no. 3, pp. 135-137, 2015. View at: Publisher
Site | PubMed
[6]
A
K Huysmans, R M Hermus, M A Edelbroek, et al. “Autoimmune hyperthyroidism
occurring late after radioiodine treatment for volume reduction of large
multinodular goiters.” Thyroid, vol. 7, no. 4, pp. 535-539, 1997. View
at: Publisher
Site | PubMed
[7]
T
W Kay, P Heyma, L C Harrison, et al. “Graves disease induced by radioactive
iodine.” Ann Intern Med, vol. 107, no. 6, pp. 857-858, 1987. View at: Publisher
Site | PubMed
[8]
Matthias
Schmidt, Eva Gorbauch, Markus Dietlein, et al. “Incidence of postradioiodine
immunogenic hyperthyroidism/Graves’ disease in relation to a temporary increase
in thyrotropin receptor antibodies after radioiodine therapy for autonomous
thyroid disease.” Thyroid, vol. 16, no. 3, pp. 281-288, 2006. View at: Publisher
Site | PubMed
[9]
L Chiovato, F Santini,
P Vitti, et al. “Appearance
of thyroid stimulating antibody and Graves’ disease after radioiodine therapy
for toxic nodular goitre.” Clin Endocrinol (Oxf), vol. 40, no. 6, pp.
803-806, 1994. View at: Publisher
Site | PubMed
[10]
Guohua Shen, Futao
Cui, Rui Huang, et al. “Graves
disease following radioiodine therapy for toxic adenoma: Clinical case report”.
Medicine (Baltimore), vol. 96, no. 45, pp. e8550, 2017. View at: Publisher
Site | PubMed
[11]
Kajetan
Zgubieński, Agnieszka Walczyk, Aldona Kowalska “Unusual case of radioactive
iodine-induced Graves’ disease with orbitopathy following total thyroidectomy
in a patient with papillary thyroid microcarcinoma.” Endokrynol Pol,
vol. 71, no. 3, pp. 277-278, 2020. View at: Publisher
Site | PubMed
[12] Mariko Aoyama, Hiromitsu Takizawa, Mitsuhiro Tsuboi, et al. “A case of metastatic follicular thyroid carcinoma complicated with Graves’ disease after total thyroidectomy.” Endocr J, vol. 64, no. 12, pp. 1143-1147, 2017. View at: Publisher Site | PubMed
[12] J Yoshimura Noh, T Mimura, M Kawano, et al. “Appearance of TSH receptor antibody and hyperthyroidism associated with metastatic thyroid cancer after total thyroidectomy.” Endocr J, 44, no. 6, pp. 855-859, 1997. View at: Publisher Site | PubMed
