Received: Tue 01, Sep 2020
Accepted: Wed 16, Sep 2020
Abstract
Objective: To analyse the clinical features of perineal endometriosis (PEM), its treatment and outcome.
Methods: Prospective, single-centre study with 13 patients with PEM who were treated between 2011-2018 at Domingo Luciani Hospital and mean followed up for 58.4 months.
Results: Mean age was 32,2 years. All cases had a history of vaginal delivery with an episiotomy. All complained of perineal pain related to the menstrual cycle; the perineal mass progressively increased in size and was tender during menstrual periods. Mean VAS was 7. 69,2% with rectal bleeding. The mean size of the lesion was 3.42 cm. CA125 levels were measured in all patients, 3 (23,1%) with abnormal range; all patients were subjected to transvaginal, endoanal ultrasonography (EUS) and FNAB before surgery. Anal sphincter (AS) involvement was demonstrated by EUS in 46.2% (6). Mean EUS pre-treatment volume 18.98 ml. First, these 6 patients received hormonal therapy based on GnRH and evaluated response. Mean EUS post-treatment volume 10.21 ml p < 0.05. Complete local excision was performed on all cases. Mean CCFIS preoperative was 2.46 and postoperative 3.01 p=0.01. No major complications or recurrences were noted.
Conclusion: PEM presents with typical clinical features when it involves the AS, it could benefit from first a hormonal therapy before surgery. EUS is a useful preoperative tool to decide what we should do. The main idea at the time of surgery is performed a complete local excision with non-touch AS, and in cases where these aren’t possible, a sphincteroplasty is mandatory with good continence results, minor complications and no recurrences.
1. Introduction
Endometriosis, which is defined as the presence of endometrial tissues outside of the uterine cavity, is one of the most common diseases in women of reproductive age, affecting 3-10% of these. In general, endometriosis can be divided into pelvic and extrapelvic sites. Endometriosis is commonest in the pelvis. The areas of the pelvis most frequently affected are the peritoneum, the ovaries, the pouch of Douglas, and the uterosacral ligament. It is very occasionally found in extrapelvic sites such as the gastrointestinal tract, pulmonary structures, the urinary system, abdominal wall, perineum or vagina, skin, and even the central nervous system. A widely accepted theory on the pathogenesis is that endometrial tissue is transplanted outside the endometrial cavity either by retrograde menstrual flow, or lymphatic or hematogenic transport [1].
Perineal endometriosis is the occurrence in the perineum of endometrial glands and stroma that respond to the hormone variations in the cycle, and when islands of endometrial tissues invade the sphincteric muscular tissue, perineal endometriosis with anal sphincter involvement occurs. Endometriosis of perineum and vulva, accounting for less than 1% of cases of surgically treated endometriosis, has been reported in the literature with the most common site being episiotomy scars [2, 3]. Similarly, seeding of endometrial tissue into wounds may be responsible for endometriosis developing in perineal scars. Since 1923, case studies of perineal endometriosis with anal sphincter involvement, a condition that has a low prevalence, have been published in the obstetric and gynecologic literature [4]. Using PubMed and ScienceDirect to search about this condition, we realized it was relatively rare disease; the diagnosis and treatment of the disease need to be further studied. This prospective study shows 13 cases of perineal endometriosis that received surgical treatment with or without pre/post hormonal therapy between January 2011 and July 2018 in Domingo Luciani Hospital. Through the following analysis of the clinical features, diagnosis, medical or surgical treatment, and prognosis of the 13 cases, we put forward our understanding of the diagnosis and treatment of the disease.
2. Methods
A single-centre prospective study was conducted at the coloproctology Unit of Domingo Luciani Hospital, Caracas, Venezuela from January 2011 to July 2018. The study protocol was approved by the local ethics research committee and was exempted from an informed consent requirement to the patients.
3. Inclusion and Exclusion Criteria
Patients with the following criteria were included: Women between 18 and 45 years with active sex life but without immediate fertility concern, who consulted for presenting clinical suspicion of perineal endometriosis and who undergo transvaginal and endoanal ultrasonography (EUS), fine-needle aspiration biopsy (FNAB) of the lesion, serum levels of CA125, and who performed surgical treatment associated or not with previous hormonal suppression and patients were able to consent to participate and attend all scheduled follow-up visits. The exclusion criteria were: Patients with current diagnosis or background of cancer, cryptoglandular anal fistula or abscess, previous anal surgery or were not able to consent to participate.
4. Study Design
Physical examination, which included a bimanual gynecologic, rectovaginal abdominal and digital rectal examination (DRE), was performed on each case. Visual Analogous pain Scale (VAS) [5] and Wexner or Cleveland Clinic Fecal Incontinence Score (CCFIS) [6] were filled. A transvaginal and three-dimensional endoanal ultrasonography (BK Medical Pro Focus 2202®) were performed before surgery to rule out pelvic lesions, measure the perianal lesion, take biopsy, and to determinate anal sphincter involvement or not (Video). Depending on this we decided if patients need hormonal treatment to try to reduce the size, or if they should be first operated. Patients’ sex, age, clinical features, localization of the lesion, relation with episiotomy, endoanal ultrasound volume and their relation with the anal sphincter, FNAB and CA125 results were examined through the review of the medical records (Table 1).
TABLE 1: Patient characteristics.
|
Characteristics |
Value |
|
Age (years)* |
32,2 (24-39) |
|
Vaginal delivery with episiotomy |
13 |
|
Parity* |
2.29 (1 – 5) |
|
Perineal pain related to the menstrual cycle |
13 |
|
Visual Analogous pain Scale (VAS)* |
7 (4-9) |
|
Palpable subcutaneous swelling |
13 |
|
Relationship to episiotomy scar |
11 |
|
Involvement anal sphincter by DRE |
3 |
|
Symptom onset (mo)* |
25.64 (4-85) |
|
CCFIS score preoperative* |
2.46 (1,391) |
|
Ca125 abnormal level (3)* |
91,8 U/ml (21,27) |
|
FNAB positive for endometriosis |
11 |
|
Ovarian endometrioma in transvaginal ultrasonography |
2 |
|
3D-EUS features |
13 |
|
Pre-treatment volume of the mass (ml)* |
18.98 (12.31– 34.14) |
|
Involvement anal sphincter by EUS |
6 |
|
First hormonal therapy with GnRH |
6 |
|
Post-treatment volume of the mass (ml)* |
10.21 ( 5.9 – 16.2) |
|
Surgery features |
13 |
|
First surgery treatment |
7 |
|
Complete Local Excision with free margins |
13 |
|
Sphincteroplasty |
4 |
|
Hormonal therapy with oral high-dose progestin |
13 |
|
CCFIS score post-operative* |
3.01 (1,472) |
|
Recurrences |
0 |
|
Follow-up (mo)* |
58.4 (27 - 92) |
*values are mean (s.d. or range). Mo: Months; DRE: Digital Rectal Examination; CCFIS: Cleveland Cleveland Clinic Fecal Incontinence Score; FNAB: Fine-Needle Aspiration Biopsy; 3D-EUS: Three-Dimensional Endoanal Ultra- Sonography; ml: millilitres; GnRH: Gonadotropin-Releasing Hormone Analogues.
Patients were divided into two groups. A group who has anal sphincter involvement in the EUS whose received hormonal therapy based on gonadotropin-releasing hormone analogues (GnRH) (Leuprolide Depot 3.75 mg intramuscular monthly for up to 6 months) and evaluating after treatment the size decrease and the possibility of surgery and another group without anal sphincter involvement who were operated without previous hormonal therapy doing complete local excision with or without sphincteroplasty. All patients received after surgery high-dose progestin-only based on Norethindrone acetate 5 mg daily for 6 months. We evaluated the possibility of total local excision, complications such as faecal incontinence and recurrence in a long follow-up. The IBM SPSS Statistics version 22.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. A value of p ≤ 0.05 was considered significant.
5. Results
Between January 2011 to July 2018, 13 consecutive women patients; mean age at the time of surgery was 32,2 (s.d.) (4,082) (range 24-39 years). The mean follow-up was 58.4 (range 27-92) months. All cases had a history of vaginal delivery with an episiotomy. All were multiparous. The mean parity was 2.29 (s.d) (1,376) (range 1-5). The mean duration of symptoms was 25.64 months (range 4-85 months). All of the patients complained of perineal pain related to the menstrual cycle; the perineal mass progressively increased in size and was tender during menstrual periods. Mean VAS 7 (range 4-9). 69,2% (9) with rectal bleeding. All of them had had a vaginal delivery with episiotomy, 10 on the right, and 3 in the median. On physical examination, in 84,6% (11), the masses were located at the episiotomy scars, and in 15,4% (2), close to rectovaginal septum but without relation with the scars. Digital rectal examination was normal in all patients, but only in 23,07% (3), involvement of the anal sphincter was suspected. The mean size of the lesion was 3.42 cm (range 1–6 cm).
6. Cancer Antigen Ca125 Levels and Transvaginal Ultrasonography
Cancer antigen (CA125) levels were measured in all patients. The mean serum level was 21.2 U/ml, 10 cases (76,9%) with normal range (4.3–34.7 U/ml) and 3 (23,1%) with abnormal range; Mean 91,8 U/ml (s.d) (21,27) (range 39.9–125.8). All patients were subjected to transvaginal ultrasonography and revealed pelvic abnormalities with concomitant ovarian endometrioma in two patients; these patients with CA125 elevated.
7. Endoanal Ultrasonography Findings
A tridimensional (3D) EUS was performed before surgery in order to determine the involvement of the anal sphincter, the volume of the mass, and to perform FNAB, these parameters were necessary to decide the conduct to follow. All patients were subjected to FNAB guided by ultrasonography and 84,6% (11) were positive for the presence of endometrial tissue, composed of glands and stroma. Anal sphincter involvement was demonstrated by EUS in 46.2% (6) (Figure 1A). The mean pre-treatment volume of the mass was 18.98 ml (s.d.) (8.65) (range 12.31– 34.14). First, these 6 patients received hormonal therapy based on Gonadotropin-releasing hormone analogues (GnRH) (Leuprolide Depot 3.75 mg intramuscular monthly for up to 6 months), and then we repeated the EUS to evaluate the response to medical treatment (Figure 1C) with mean post-treatment volume 10.21 ml (range 5.9-16.2) p < 0.05 and 4 patients with involvement of anal sphincter.
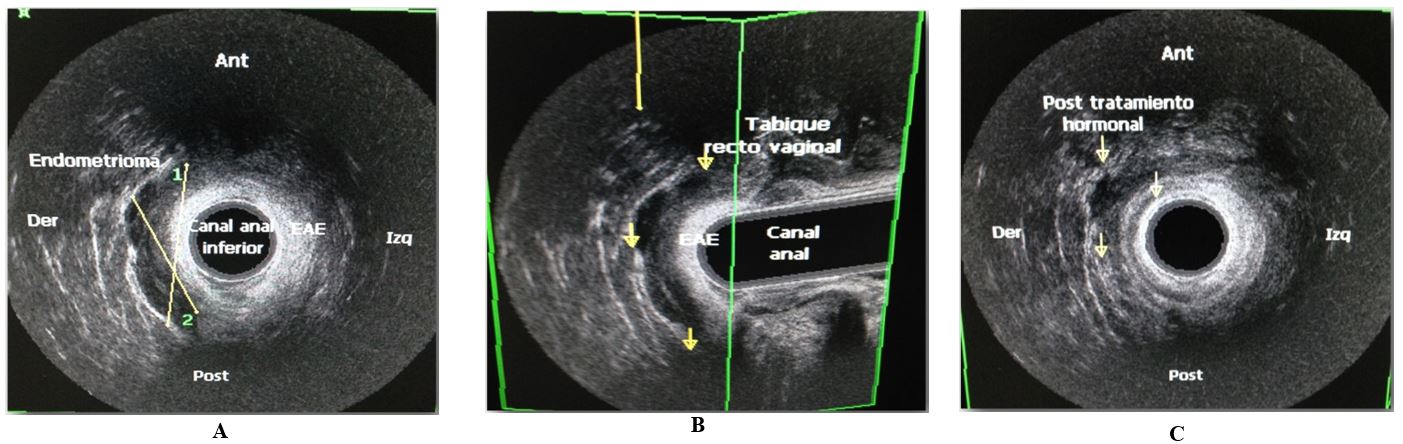
8. Surgery Details
Surgical treatment was performed in all cases. Complete local excision was performed on 100 % cases, during which a circumferential and deep surgical margin of 0.5–1.0 cm outside the edge of perianal mass was resected. Primary anal sphincteroplasty was performed in 4 cases using 2-0 Polydioxanone suture with overlapping technique. The perineal wound was closed in all cases by planes using intermittently 3–0 vicryl sutures and/or polypropylene in the skin. A penrose drain was left for 3 to 5 days (Figures 2 & 3). The stitches were removed 2 weeks later approximately. All definitive biopsies reported endometriosis with free margins.
9. Clinical Continence and Complications of the Procedure
No important changes in anal continence. Mean Cleveland Clinic Fecal Incontinence Score (CCFIS) preoperative was 2.46 (s.d) (1,391) (range 0–5) and mean postoperative 3.01 (s.d) (1,472) (range 0–6); p = 0.01. No major complications were reported in our series, only two patients, one with a wound infection treated with antibiotics and delayed closure and another case with a short perineum-vaginal fistula that was later corrected with fistulotomy and primary closure. All patients took after surgery high-dose progestin-only based on Norethindrone acetate 5 mg daily for 6 months. Perineal pain relief completely after surgical recovery. No recurrences were reported in the follow-up.
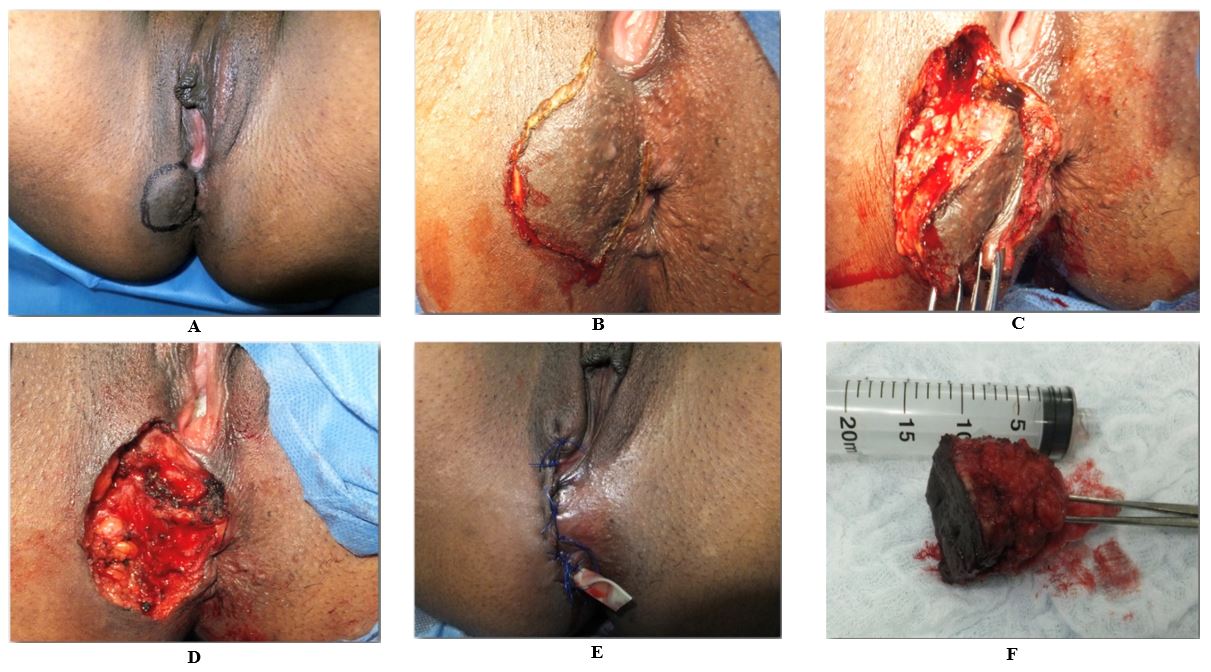
A) Lesion marking. B) Incision. C) Dissection and visualization of chocolate liquid, typical of endometriosis lesions. D) Surgical wound with partial resection of the EAS. E) Closure by planes, previous sphincteroplasty and placement of a penrose drain. F) Surgical specimen of approx. 5 x 5 cm.
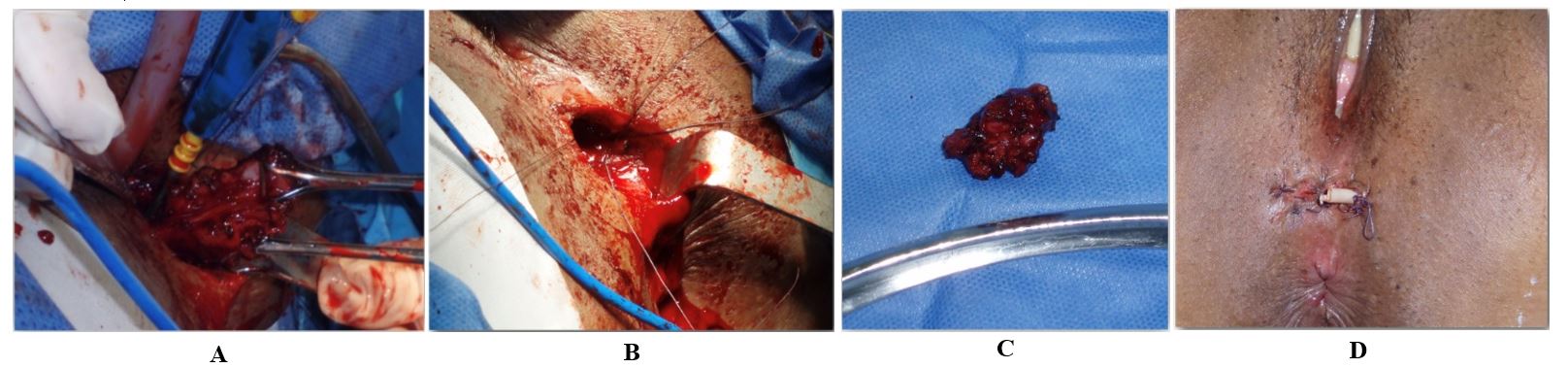
10. Discussion
Functioning endometrial tissue located outside of the uterine cavity is defined as endometriosis [1]. Cutaneous endometriosis is rare and mostly occurs in the abdominal wall, usually developing at the site of a caesarian scar. Perineal and vulvar lesions are rarer; the iatrogenic transplantation of endometrial cells via an episiotomy scar appears to be the mechanism involved [7], and our cases support this hypothesis. Diagnosis of perineal endometriosis is usually highly suggestive from the history and examination alone. Diagnostic clues include intermittent enlargement and tenderness of the lesion around the time of menses. Most of the patients present with a tender, palpable subcutaneous swelling near or within the surgical scar. The cyclic nature of the swelling and pain, which worsen at the time of menstruation, and a frequently reported history of gynecologic or rarely non-gynecologic abdominal surgery, are nearly pathognomonic [8], these have been in corresponding with clinical features in our cases; however, we exclude patients with anorectal surgery because of the possibility of produce for example granulomas in the scars. The differential diagnosis of such patients includes granulomas, anal fistula, abscesses, atheroma, hidradenitis, anal melanoma, etc. The tools used to aid diagnosis are controversial. Tumor markers are not very sensitive for extraovarian lesions, even in malignant cases. Levels of CA125 are normal in almost half of patients with extraovarian lesions but are normal in only 15.38% of cases with ovarian endometriosis [9].
In our study, serum CA125 levels were elevated in three cases (23.1%); of those, two (15.3%) also had ovarian endometrioma visualized by transvaginal ultrasonography and the other one (7.6%) had anal sphincter involvement, for this reason transvaginal and endoanal ultrasonography should be performed in all patients with perianal endometriosis, especially if serum CA125 levels are elevated. Malignant degeneration of cutaneous endometriosis is extremely rare, representing 0.3-1% of surgical scars, and its origin is still unclear [10]; for this rare but feasible possibility, we performed FNAB on all the patients reporting benign in 100% of cases: 84,6% (11) were positive for the presence of endometrial tissue, composed of glands and stroma, and two (15.4%) reported unspecific cells inflammatory. EUS has been defined as the gold standard for assessment of the anal canal [11, 12].
3D EUS with high spatial resolution allows multiplanar evaluation and good tissue characterization of the sphincter muscles, the perianal tissues, and the rectovaginal septum [13]. In addition, transperineal ultrasonography (TPUS) allows the assessment of the vascularity by using power doppler [14]. In our study, we don´t use TPUS because of don´t have this kind of transductor, but we use EAUS and using this, like Santoro et al., anal endometriosis was most frequently visualized as a hypoechoic or mixed echogenicity lesion, with areas of microcalcification or small cysts, not well delimited at the same time the relationship of the lesion with the anal sphincters and the rectal wall could be evaluated [15]; with this important tool in our study, we divided the sample into two groups to decide if the patient needed hormonal therapy before surgery or not depending on anal sphincter involvement, demonstrating a relevant size volume lesion decrease with this therapy and the possibility of doing complete local excision with sphincteroplasty in 4 patients of 6 who received hormonal suppression. Some authors have suggested that in younger patients, complete local excision with sphincteroplasty may be optimal, obviating the need for additional therapy. And in older patients who were closer to menopause, narrow or incomplete local excision with subsequent hormonal therapy could be advantageous (when endometriosis tends to regress) to lessen the risk of incontinence with sphincter resection [16].
Despite the fact that our sample was made up of young patients, we preferred to give hormonal therapy in cases involving the anal sphincter with the intention of avoiding performing the sphincteroplasty or doing the least possible damage to the sphincter, knowing that in the future, such damage could have consequences in anal continence. Our study is the only one in the literature that evaluates pre- and post-surgical anal continence in this entity using a scale, showing no changes in this parameter in a long follow-up; also there is no consensus in the literature about the advantages when pre-operative medical therapy is given and not given. However, like in the study of Na Chen et al., we recommend the use of preoperative hormone therapy and the reasons are as follows: preoperative hormone therapy could reduce the size of the endometrioma and make the boundaries of these lesions clearer, thus facilitating the complete excision of the lesions and reducing damage to surrounding tissues [17]; we also decided to administer to all patients after surgery a high dose of progestin based on norethindrone acetate 5 mg daily for 6 months with the intention of reducing local recurrence, which in our series has been nil; this is in contrast with the variability rate of recurrence reported in other studies [3].
11. Conclusion
Perineal endometriosis is a rare entity with typical clinical features; however, there are no work protocol established in the literature. In our short experience, endometriosis that involves the anal sphincter could be benefitted from first a hormonal therapy before surgery that allows decrease volume of the lesion. Endoanal ultrasound is a useful preoperative tool to decide what we should do. The main idea at the time of surgery is performing a complete local excision without touching the anal sphincter, and in cases where these aren’t possible, a sphincteroplasty is mandatory; these have, in our experience, shown good continence results, minor complications and no recurrences in a long follow-up.
Conflicts of Interest
None.
Funding
None.
REFERENCES
- Linda C Giudice, Lee C Kao “Endometriosis.” Lancet, vol. 364, no. 9447, pp. 1789-1799, 2004. View at: Publisher Site | PubMed
- Kaei Nasu, Mamiko Okamoto, Masakazu Nishida, et al. “Endometriosis of the perineum.” J Obstet Gynecol Res, vol. 39, no. 5, pp. 1095-1097, 2013. View at: Publisher Site | PubMed
- Zhu L, Lang J, Wang H, et al. “Presentation and management of perineal endometriosis.” Int J Gynaecol Obstet, vol. 105, no. 3, pp. 230-232, 2009. View at: Publisher Site | PubMed
- Schickele M “Les pseudolipomes du ligament large.” Bull Mem Soc Anat Paris, vol. 20, pp. 603-605, 1923.
- Nicolas Bourdel, João Alves, Gisele Pickering, et al. “Systematic review of endometriosis pain assessment: how to choose a scale?” Human Reprod Update, vol. 21, no. 1, pp. 136-152, 2014. View at: Publisher Site | PubMed
- J M Jorge, S D Wexner “Etiology and management of fecal incontinence.” Dis Colon Rectum, vol. 36, no. 1, pp. 77-97, 1993. View at: Publisher Site | PubMed
- Muzeyyen Gunes, Fulya Kayikcioglu, Esmen Ozturkoglu, et al. “Incisional endometriosis after cesarean section, episiotomy and other gynecologic procedures.” J Obstet Gynaecol Res, vol. 31, no. 5, pp. 471-475, 2005. View at: Publisher Site | PubMed
- Nicola Cinardi, Salvatore Franco, Danilo Centonze, et al. “Perineal scar endometriosis ten years after Mile’s procedure for rectal cancer: case reportand review of the literature.” Int J Surg Case Rep, vol. 2, no. 6, pp. 150-153. View at: Publisher Site | PubMed
- Tito Silvio Patrelli, Roberto Berretta, Salvatore Gizzo, et al. “CA 125 serum values in surgically treated endometriosis patients and its relationships with anatomic sites of endometriosis and pregnancy rate.” Fertil Steril, vol. 95, no. 1, pp. 393-396, 2011. View at: Publisher Site | PubMed
- G Chene, C Darcha, P Dechelotte, et al. “Malignant degeneration of perineal endometriosis in episiotomy scar, case report and review of the literature.” Int J Gynecol Cancer, vol. 17, no. 3, pp. 709-714, 2007. View at: Publisher Site | PubMed
- Bliss DZ, Mellgren A, Whitehead WE, et al. “Assessment and conservative management of faecal incontinence and quality of life in adults.” Paris: ICUD- EAU, vol. 16, pp. 1443-1486, 2013.
- G González Longoria, R R Mejía Ovalle, E Salinas Aragón, et al. “Perineal endometriosis with anal external sphincter involvement: a case-report.” Rev Gastroenterol Mex, vol. 76, no. 2, pp. 173-177. View at: PubMed
- Giulio A Santoro, Bjørn Fortling “The advantages of volume rendering in three-dimensional endosonography of the anorectum.” Dis Colon Rectum, vol. 50, no. 3, pp. 359-368, 2007. View at: Publisher Site | PubMed
- Chiara Del Frate, Rossano Girometti, Marco Pittino, et al. “Deep retroperitoneal pelvic endometriosis: MR imaging appearance with laparoscopic correlation.” Radiographics, vol. 26, no. 6, pp. 1705-1718, 2006. View at: Publisher Site | PubMed
- M Kołodziejczak, I Sudoł Szopińska, G A Santoro, et al. “Ultrasonographic evaluation of anal endometriosis: report of four cases.” Tech Coloproctol, vol. 18, no. 11, pp. 1099-1104, 2014. View at: Publisher Site | PubMed
- L S Dougherty, T Hull “Perineal endometriosis with anal sphincter involvement: report of a case.” Dis Colon Rectum, vol. 43, no. 8, pp. 1157-1160, 2000. View at: Publisher Site | PubMed
- Na Chen, Lan Zhu, Jinghe Lang, et al. “The clinical features and management of perineal endometriosis with anal sphincter involvement: a clinical analysis of 31 cases.” Hum Reprod, vol. 27, no. 6, pp. 1624-1627, 2012. View at: Publisher Site | PubMed
