Received: Mon 21, Sep 2020
Accepted: Mon 12, Oct 2020
Abstract
The paraneoplastic syndromes occur in upto 28% of patients with hepatocellular carcinoma (HCC). Among them, erythrocytosis is reported as just 3.9 % of frequency. We describe a 59-year-old male patient with a large HCC in a non-cirrhotic liver presenting with erythrocytosis. Four phlebotomies were performed before radical surgical resection. Extended right hepatectomy was undertaken, proving a unifocal 10.2 X 13 X 6.5 cm, extensively necrotic HCC. There were no postoperative complications, and the patient was discharged on the 6th postoperative day. Adjuvant treatment with thalidomide was indicated for 36 months. We observed a progressive decrease in haemoglobin, haematocrit, EPO, and AFP to normal levels after the surgical procedure. A periodic follow-up with laboratory parameters and CT scan was done for 46 months with no evidence of relapses, until now. This case highlights a rare manifestation of advanced liver cancer; the first documented paraneoplastic erythrocytosis in a large HCC case in Chile was successfully treated by radical liver resection and adjuvant thalidomide.
1. Introduction
Hepatocellular carcinoma (HCC) is the most frequent malignant liver tumor worldwide, ranking at the fourth and sixth positions in terms of mortality (>781,000 deaths) and incidence (>840,000 cases), respectively [1]. In Chile, 1,582 new cases were diagnosed with 1,448 deaths by 2018, becoming the eighth cancer mortality cause [1]. Nowadays, the epidemiological landscape of HCC has suffered significant changes. While chronic viral hepatitis and excessive alcohol consumption have long been described as the main risk factors for HCC, non-alcoholic steatohepatitis (NASH), following the worldwide epidemic of obesity and type 2 diabetes, has become a growing cause of HCC in the western countries [2].
Paraneoplastic syndromes in HCC are not uncommon. They can be found in about 20-28% of cases [3, 4]; most frequent are hypercholesterolemia (24.5%), hypercalcemia (5.3%), and erythrocytosis (3.9%) [5]. Most HCC patients with paraneoplastic syndrome have massive tumors and increased alpha-fetoprotein (AFP) levels in peripheral blood [3, 4]. The erythrocytosis mechanism is not entirely understood, but erythropoietic activity has been demonstrated in HCC patient sera, and erythropoietin (EPO) has been found by immunohistochemical staining in tumor cells but not in normal hepatocytes on surgical specimens [4]. Until now, resection and liver transplantation are the main curative treatments, although a small number of patients achieve to benefit.
Herein, we describe a case of erythrocytosis caused by a massive HCC in a patient with NASH successfully treated by a liver resection with 46 months follow-up without evidence of recurrence.
2. Clinical Case
A 59-year-old male patient was referred to our centre because of perioral cyanosis and asthenia. No weight loss was noted. Laboratory studies showed a red cell count elevated, with a haemoglobin level of 20.3 g/dl, a haematocrit of 62%, and serum erythropoietin (EPO) of 115 mU/ml (normal range 2.6-18.5 mU/ml). Past medical history was uneventful, although a fatty liver was described on ultrasound some years ago. Abdominal ultrasound and MRI scan found a bulky mass in the right hepatic lobe measuring 10.2 x 15.6 x 6.2 cm. The liver showed moderate diffuse steatosis, without signs of cirrhosis (Figure 1). An F18-FDG PET-CT demonstrated marked glucose metabolic activity within the right liver mass with no evidence of distant metastatic foci (Figure 2). Serological tumor markers revealed a raised alpha-fetoprotein level of 36,210 ng/mL, with a CA 19-9 slightly increased (60.8 IU/mL, normal value < 34). Serological tests for hepatitis B and C virus were negative.
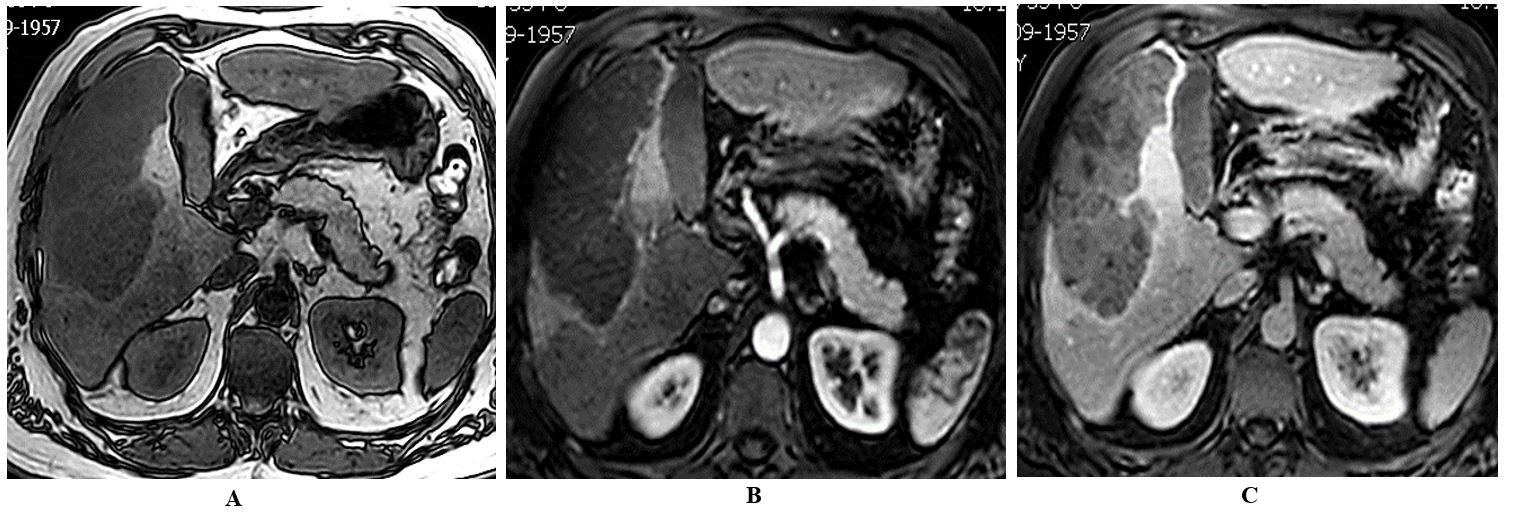
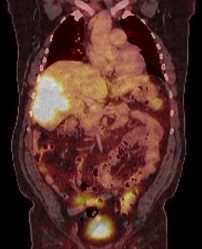
We programmed four phlebotomies to reduce the thromboembolic risk achieving a haemoglobin level of 14 g/dl with a haematocrit of 45% before a radical resection of the tumor with an open extended right hepatectomy was performed. No RBC packet was required during an uneventful surgical liver resection. There were no postoperative complications, and the patient was discharged on the 6th postoperative day.
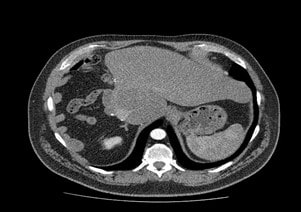
Pathological analysis of the surgical specimen, which weighed 1,773 g, showed a unique expansive tumor measuring 10.2 X 13 X 6.5 cm, extensively necrotic. Formalin-fixed tissue was embedded in paraffin, and the sections were firstly stained with Haematoxylin and Eosin (HE). Immunohistochemistry confirmed a moderately differentiated hepatocellular carcinoma confined to the liver lobe. No satellite nodules were found, and microscopic vascular invasion was present. Surgical margins and hilar lymph nodes were negatives. TNM Staging (IUCC, 7° edit) [6] was pT2 N0 M0. The surrounding liver tissue histopathological examination showed moderate steatosis with steatohepatitis and steatofibrosis with some thin septa.
Serum levels of haemoglobin, EPO, and AFP returned to normal levels some days after the liver resection and closely monitoring of these variables was undertaken every six months during follow-up. We decide to provide him an antiangiogenic therapy prescribing thalidomide 300 mg/day for three years. Yearly CT scans and MRI have not revealed recurrence after 46 months follow-up period (Figure 3). The liver function tests were normal, and no signs of chronic liver disease or portal hypertension were found.
3. Discussion
Paraneoplastic erythrocytosis is a rare manifestation of advanced HCC. Regression of hyperhemoglobinemia after radical oncological resection proved the connection between the two phenomena in our case. Of note, erythrocytosis was the first abnormality found in the described patient, and HCC diagnosis was made after appropriate work out of elevated levels of haemoglobin.
There are different causes of erythrocytosis that can be classified as primary and secondary. Primary erythrocytosis is the EPOR mutation, a primary congenital entity, and also polycythemia vera, an acquired disease. On the other hand, the acquired secondary etiologies are divide into two groups, those where EPO increase with hypoxia, such as chronic pulmonary disease, hypoventilation syndromes, right to left cardiopulmonary communication, carbon monoxide chronic intoxication, renal artery stenosis and polycystic kidney disease, and without hypoxia, like EPO producing tumors as clear and chromophobe cell renal, hepatocellular, adrenal and parathyroid carcinomas and cerebellar hemangioblastoma among others [7-11].
During the fetal period, the liver is the largest organ secreting EPO. Thus, poor or undifferentiated HCC can liberate this hormone as a de-differentiating tumor cell function. It has been shown that HCC produces EPO by immunohistochemistry, where strong cytoplasmic stains in tumor tissue are seen, but not in non-tumor liver cells [4]. Some of these neoplastic cells produce alpha-fetoprotein (AFP) as well [4, 11]. Local hypoxia in fast-growing tumors could explain EPO secretion in large HCC. A recent report shows the experimental correlation between hypoxic micro-environment and the Hypoxia Inducing Factor (HIF) gene expression inducing EPO secretion in human HCC cells and mice HCC models [12].
Some reports show that patients with NASH-HCC generally have a more advanced presentation of HCC at diagnosis [13, 14], as our patient. A cohort study by Mittal et al. revealed that in comparison to HCV-related HCC, fewer cases of NASH-HCC were diagnosed in early HCC stages or Barcelona Clinic Liver Cancer (BCLC) stage A (5.8% vs. 15.7%, p = 0.04). Also, more NASH-HCC patients were diagnosed in terminal stages or BCLC stage D (19.2% vs. 16.1%, p = 0.04) [14]. In many countries, advanced hepatocellular carcinoma usually presents as abdominal pain or mass with anorexia and weight loss, especially in rural areas of sub-Saharan Africa and Southeast Asia, where radical treatment is scarcely done [4, 5].
Moreover, different studies show that up to 28% of patients with massive HCC have paraneoplastic manifestations. More specifically, up to 3-15% of them have erythrocytosis, although the prevalence varies between studies [4, 5]. Additionally, a correlation has been found between paraneoplastic syndrome and higher levels of AFP. Paraneoplastic HCC manifestations are associated with more advanced stages and larger tumor size [5]. Then, they are adverse prognosis factors in HCC patients undergoing surgical resection.
Radical liver resection is the only definitive treatment for these advanced HCC cases. Liver transplantation (LT), probably the best curative treatment for HCC, is constrained to some restrictive criteria [15, 16], that a priori, leave these patients outside of LT indication because of the more aggressive biology and the larger size of these tumors. Most huge HCC with paraneoplastic symptoms are grounded on no cirrhotic livers, although chronic inflammation, as in this case, is frequently observed on pathology assessment [17]. Then, it does not fix the actual indications of liver replacement. Therefore, surgical resection remains a critical primary treatment option for these large HCCs.
Because of the frequent recurrence during HCC patient's follow-up, efforts to find an effective adjuvant systemic therapy are justified [18]. Sorafenib, an oral multikinase inhibitor blocking tumor angiogenesis and progression, has been considered the standard of care for patients with advanced unresectable HCC since 2007 [19]. However, it failed to demonstrate a benefit as an adjuvant treatment in resected or ablated HCC [20]. Many other trials have been designed with new or combination of agents, where interferon therapy seems to have a promising role in reducing relapse and prolong survival after surgery [21]. Thalidomide, an antiangiogenic agent, has been shown to stabilize advanced HCC, with reasonable tolerability, with some cases reported with complete remission [22-24]. There is one on-going clinical trial to assess thalidomide as an adjuvant treatment after HCC curative resection with no definitive results yet reported [25]. We decide to prescribe it to our patient because of the predicted poor prognosis of his HCC. At 46 months follow-up, no recurrences have been observed.
REFERENCES
- Global Cancer Observatory of the International Agency for Research on Cancer. (WHO) Globocan website (https://gco.iarc.fr/today/online-analysis), 2020.
- Lequoy M, Gigante E, Couty JP, et al. “Hepatocellular carcinoma in the context of non-alcoholic steatohepatitis (NASH): recent advances in the pathogenic mechanisms.” Horm Mol Biol Clin Investig, vol. 41, no. 1, 2020. View at: Publisher Site | PubMed
- Chu CW, Hwang SJ, Luo JC, et al. “Manifestations of hypercholesterolaemia, hypoglycaemia, erythrocytosis and hypercalcaemia in patients with hepatocellular carcinoma: report of two cases.” J Gastroenterol Hepatol, vol. 14, no. 8, pp. 807-810, 1999. View at: Publisher Site | PubMed
- Matsuyama M, Yamazaki O, Horii K, et al. “Erythrocytosis caused by an erythropoietin-producing hepatocellular carcinoma.” J Surg Oncol, vol. 75, no. 3, pp. 197-202, 2000. View at: Publisher Site | PubMed
- Chang PE, Ong WC, Lui HF, et al. “Epidemiology and Prognosis of Paraneoplastic Syndromes in Hepatocellular Carcinoma.” ISRN Oncol, vol. 2013, pp. 684026, 2013. View at: Publisher Site | PubMed
- Sobin LH, Gospodarowicz MK, Wittekind C “TNM Classification of Malignant Tumours. (IUCC International Union Against Cancer), 7th Edition.” Wiley Blackwell, 2011.
- McMullin MF “Investigation and Management of Erythrocytosis.” Curr Hematol Malig Rep, vol. 11, no. 5, pp. 342-347, 2016. View at: Publisher Site | PubMed
- Cannavo L, Zirrilli G, Lima M, et al. “Erythrocytosis as the first manifestation of adrenal carcinoma.” Pediatr Blood Cancer, vol. 66, no. 6, pp. e27685, 2019. View at: Publisher Site | PubMed
- Guo R, Liang Y, Yan L, et al. “Erythrocytosis caused by giant chromophobe renal cell carcinoma: a case report indicating a 9-year misdiagnosis of polycythemia vera.” Chin J Cancer, vol. 36, no. 1, pp. 72, 2017. View at: Publisher Site | PubMed
- Martínez A, González A, Ropero P, et al. “Differential diagnosis of erythrocytosis. Hemoglobins with high oxygen affinity.” Real Academia Nacional de Medicina de España, vol. 137, no. 1, pp. 35-43, 2020.
- Kew MC “Paraneoplastic Phenomena in Patients with Hepatocellular Carcinoma.” J Liver Res Disord Ther, vol. 2, no. 1, pp. 9-12, 2016. View at: Publisher Site
- Miao S, Wang SM, Cheng X, et al. “Erythropoietin promoted the proliferation of hepatocellular carcinoma through hypoxia induced translocation of its specific receptor.” Cancer Cell Int, vol. 17, pp. 119, 2017. View at: Publisher Site | PubMed
- Piscaglia F, Svegliati Baroni G, Barchetti A, et al. “Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study.” Hepatology, vol. 63, no. 3, pp. 827-838, 2016. View at: Publisher Site | PubMed
- Mittal S, Sada YH, El Serag HB, et al. “Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population.” Clin Gastroenterol Hepatol, vol. 13, no. 3, pp. 594-601, 2015. View at: Publisher Site | PubMed
- Mazzaferro V, Regalia E, Doci R, et al. “Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis.” N Engl J Med, vol. 334, no. 11, pp. 693-699, 1996. View at: Publisher Site | PubMed
- Yao FY, Ferrell L, Bass NM, et al. “Liver transplantation for hepatocellular carcinoma. Comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh Modified TNM criteria.” Liver Transpl, vol. 8, no. 9, 765-774, 2002. View at: Publisher Site | PubMed
- Desai A, Sandhu S, Lai JP, et al. “Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review.” World J Hepatol, vol. 11, no. 1, pp. 1‐18, 2019. View at: Publisher Site | PubMed
- Akateh C, Black SM, Conteh L, et al. “Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma.” World J Gastroenterol, vol. 25, no. 28, pp. 3704‐3721, 2019. View at: Publisher Site | PubMed
- Llovet JM, Ricci S, Mazzaferro V, et al. “Sorafenib in advanced hepatocellular carcinoma.” N Engl J Med, vol. 359, no. 4, pp. 378-390, 2008. View at: Publisher Site | PubMed
- Bruix J, Takayama T, Mazzaferro V, et al. and Storm investigators. “Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial.” Lancet Oncol, vol. 16, no. 13, pp. 1344-1354, 2015. View at: Publisher Site | PubMed
- Zhu GQ, Shi KQ, Yu HJ, et al. “Optimal adjuvant therapy for resected hepatocellular carcinoma: a systematic review with network meta-analysis.” Oncotarget, vol. 6, no. 20, pp. 18151-18161, 2015. View at: Publisher Site | PubMed
- Patt YZ, Hassan MM, Lozano RD, et al. “Thalidomide in the treatment of patients with hepatocellular carcinoma: a phase II trial.” Cancer, vol. 103, no. 4, pp. 749-755, 2005. View at: Publisher Site | PubMed
- Chen Y, Yen H, Chou K, et al. “Thalidomide-based multidisciplinary treatment for patients with advanced hepatocellular carcinoma: A retrospective analysis”. World J Gastroenterol, vol. 18, no. 5, pp. 466-471, 2012. View at: Publisher Site | PubMed
- Chuah B, Lim R, Boyer M, et al. “Multi-centre phase II trial of Thalidomide in the treatment of unresectable hepatocellular carcinoma.” Acta Oncol, vol. 46, no. 2, pp. 234-238, 2007. View at: Publisher Site | PubMed
- “Adjuvant Therapy with Thalidomide After Curative Resection of Hepatocellular Carcinoma”, 2020. View at: Publisher Site
