Received: Sat 11, Jul 2020
Accepted: Fri 24, Jul 2020
Abstract
Introduction: Colorectal cancer risk stratification traditionally focuses on tumor node metastasis staging. Seemingly equivalent tumors can differ unpredictably in prognosis; more sophisticated quantification and stratification methods are required to identify tumors with a high likelihood of becoming metastatic. Hypoxia (low oxygen concentration) is associated with aggressive phenotypes and poor prognosis. Hypoxia is associated with treatment resistance consequently there is an unmet clinical requirement to develop personalised treatment based on hypoxia. Positron emission tomography/computed tomography (PET/CT) imaging can non-invasively detect hypoxic tumors. [18F]Fluoroazomycin arabinoside ([18F]FAZA) is a leading hypoxia PET/CT radiotracer, and uptake is associated with lower disease free survival.
Case Report: A 78-year-old man, diagnosed with a localised colorectal cancer, underwent [18F]FAZA PET/CT imaging pre-operatively. This confirmed hypoxic regions in the tumor with correlation demonstrated with carbonic anhydrase IX (CAIX) immunohistochemistry (IHC). He underwent a right hemicolectomy. The pathological staging for his colorectal cancer predicted a good outcome; thus, he did not receive adjuvant chemotherapy. The patient subsequently developed early metastatic disease with two lung metastases, which were resected by thoracotomy and wedge resection. He continues on follow up at present with no evidence of recurrent disease.
Conclusion: Hypoxia can be an important marker in colorectal cancer when determining risk and prognosis. We present evidence of clinical correlation of FAZA uptake and CAIX IHC in colorectal cancer, a key aspect in FAZA tracer validation. PET/CT potentially provides a specific tool for stratification for hypoxia-related treatment modification and development of hypoxia biomarkers.
1. Introduction
Risk stratification for colorectal cancer has traditionally used the tumor node metastasis (TNM) staging system. Seemingly equivalent tumors can differ unpredictably in prognosis and treatment response; more sophisticated quantification and stratification methods are required to identify tumors with a high likelihood of progressing or becoming metastatic. The role of non-anatomical factors is becoming more prominent, including lymphocytic infiltration, venous invasion, tumor deposits, budding, circumferential margin status, distribution of nodal metastases and genetic characteristics [1]. Four transcriptional consensus molecular subtypes (CMS) have been proposed, each associated with distinct histopathological features including microsatellite instability (MSI) status, CpG island methylator phenotype, somatic copy number alterations, BRAF mutations, KRAS mutations, and immune infiltration [2].
Hypoxia (low oxygen concentration) is an additional characteristic for stratification because it is associated with treatment resistance, aggressive phenotypes and poor prognosis in most solid tumor types. By using radioactive molecules that are selectively retained in hypoxic cells, positron emission tomography/computed tomography (PET/CT) imaging can non-invasively detect hypoxic regions. PET/CT can visualize the heterogeneous nature of O2 levels across the entire tumor, quantify the hypoxic region(s), be utilised at multiple time points, and permit the effect of therapy on oxygenation to be assessed. Accordingly, its potential application in assisting the development of treatment strategies such as radiotherapy dose painting and hypoxia-targeted pharmacotherapies is being precisely investigated. Equally, it has the potential to help identify hypoxia gene expression signatures that may aid the assessment of prognosis or development of tailored therapeutic options. As yet, there is limited research into the use of hypoxia PET/CT in colorectal cancer, with more widespread development in head and neck (H&N), lung and glioblastoma tumors [3]. Given the documented treatment resistance related to hypoxia, there is a clear and unmet clinical requirement to develop a personalised treatment plan based on hypoxia.
[18F]Fluoroazomycin arabinoside ([18F]FAZA) is one of the leading hypoxia PET/CT radiotracers [3]. [18F]FAZA has been studied in H&N and non-small cell lung cancer (NSCLC) patients, with clear uptake seen [4]. Increased uptake in H&N patients is strongly associated with lower disease-free survival [5]. [18F]FAZA has potential advantages over another leading hypoxic tracer, [18F]fluoromisonidazole ([18F]FMISO), due to lower background signal and shorter time (1-2h) required between injection and imaging. One study has previously demonstrated feasibility for [18F]FAZA uptake in colorectal cancer [6]. A key aspect of tracer validation is the correlation of hypoxia PET/CT data with immunohistochemistry (IHC) hypoxia assessment. Correlation between [18F]FMISO and standard hypoxia IHC markers such as Hypoxia Inducible Factor 1a (HIF1α) expression in H&N patients has been demonstrated [7, 8]. However, these results have not yet been convincingly replicated with [18F]FAZA PET/CT in the clinical setting [9, 10].
We present a case where anatomical and pathological staging predicted a good outcome. As part of a pilot study, the patient had pre-operative [18F]FAZA PET/CT imaging, which suggested hypoxic regions in the tumor, confirmed with carbonic anhydrase IX (CAIX) staining on IHC. The patient subsequently developed early metastatic disease, suggestive of a more aggressive disease process than anticipated from the initial favourable pathology. This case highlights that hypoxia can be an important marker in colorectal cancer when determining risk and prognosis. Furthermore, the correlation of FAZA PET/CT uptake with hypoxia IHC markers such as CAIX can be demonstrated in a case of colorectal cancer. Hypoxia PET/CT potentially provides a specific tool for stratification for hypoxia-related treatment modification.
2. Case Report
A 78-year-old male presented with fatigue and dizziness, postural hypotension, diarrhoea, and bleeding per rectum (PR), associated with iron deficiency anaemia. He had no other significant history, including no altered bowel habit, pain, dysphagia or vomiting. Past medical history included hypertension and diet-controlled type 2 diabetes mellitus. There was no significant family history, and previous colorectal screening was negative. He was fully independent of activities of daily living with World Health Organisation (WHO) performance status 0. He was initially reviewed in the clinic, and an outpatient colonoscopy and upper gastrointestinal endoscopy (UGIE) was arranged. There were no significant findings on initial examination. His routine blood tests confirmed iron deficiency anaemia with ferritin 12.6. However, he was subsequently admitted with postural hypotension, diarrhoea and fresh per rectum (PR) bleeding, associated with anaemia. This was managed conservatively with a transfusion of 2 red cell concentrates (RCC).
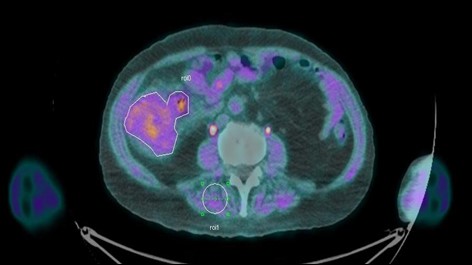
Image demonstrating [18F]FAZA uptake (purple) within colorectal primary (white outline), consistent with a hypoxic tumor. Tumor SUV max was 2.65, with a T:M ratio of 1.80 measured from baseline of skeletal muscle (white circle) and baseline uptake of adjacent bowel.
A CT staging scan confirmed a caecal mass, measuring 9 x 7 x 7.5cm with multiple enlarged local lymph nodes but no evidence of distant metastases. He subsequently underwent a colonoscopy, which confirmed a caecal tumor, and biopsy confirmed high-grade dysplasia with features suspicious of adenocarcinoma. Given the extent of disease on imaging and colonoscopy, the gentleman was reviewed and considered for a right hemicolectomy. As part of a pilot clinical study, the day prior to surgery, he underwent a [18F]FAZA PET/CT scan, which confirmed radiotracer uptake within the primary tumor, suggesting the tumor contained hypoxic regions. Images were examined using the PBAS PET image analysis suite (PMOD Technologies, Zurich, CH). Regions of interest were defined by a trained Radiologist and analysed in conjunction with the PET uptake data for the creation of time-activity curves and uptake values. Regions were defined around the tumor, an adjacent area of normal bowel and an area of skeletal muscle. The tumor maximum standardised uptake value (SUV) was 2.65, with a tumor:muscle (T:M) ratio of 1.80 (Figure 1). The definition of hypoxia is generally accepted in the literature as tumor:background ratio of ≥ 1.4 [3].
The patient underwent a right hemicolectomy, with the tumor clearly identified and resection margins macroscopically clear. Pathological examination of the formalin-fixed specimen confirmed a large 65mm polypoidal mass within the caecum, a moderately differentiated adenocarcinoma, invading through the muscularis propria, with no involved lymph nodes identified in an examination of 29 nodes and no extramural venous invasion. The final pathology was Dukes B, pT3 pN0, V0 with complete resection (R0). Additional biomarker profile demonstrated a Gly13Asp KRAS mutation, BRAF wild type and MSS stable. Immunohistochemistry (IHC) analysis of the tumor samples for the intrinsic hypoxic marker carbonic anhydrase nine (CAIX) [11], demonstrated focal positivity, confirming the hypoxic nature of the tumor identified on FAZA PET/CT (Figure 2). The patient was placed on routine follow up with no requirement for adjuvant chemotherapy.
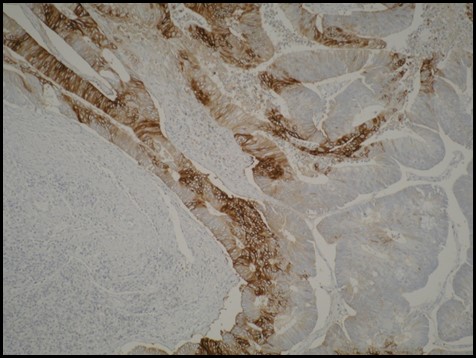
IHC analysis of the primary tumor demonstrating patchy positivity (brown) and confirming the hypoxic nature of the tumor. IHC for CAIX was performed on a section from a formalin fixed wax embedded tumor block.
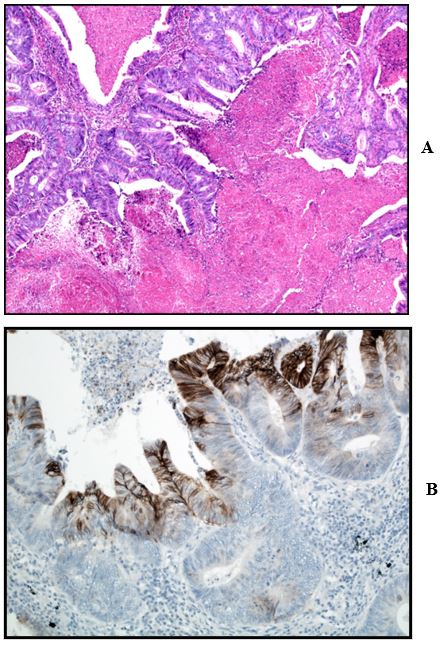
One year after his initial surgery, a routine follow-up CT scan, unfortunately, demonstrated 2 new lesions within the right lung (RUL and RML), likely to represent metastatic disease. The two lung lesions were confirmed to be avid on [18F]fluorodeoxyglucose (18F-FDG) PET/CT scan, but there was no evidence of local recurrence or other metastatic disease. He, therefore, underwent a right thoracotomy and wedge resection of the two lung nodules. In both lesions, histology confirmed a multinodular deposit of enteric pattern adenocarcinoma, in keeping with metastatic disease from the patient's previous caecal adenocarcinoma. Histology confirmed areas of necrosis and patchy CAIX positivity (Figures 3A & 3B). The gentleman remains on follow up, is clinically well and his most recent CT scan is clear of recurrence.
3. Discussion
Colorectal cancer prognostication traditionally arises from the TNM staging system, with additional risk factors assessed including extramural venous invasion, to ascertain if a patient requires adjuvant chemotherapy [1]. However, it is apparent that the outcome of patients may vary considerably within the same TNM stage classification. This was clearly demonstrated in relation to this case, where the patient developed metastases unexpectedly given the original relatively low risk predicted by the histopathological assessment. These variations and unexpected results in certain patients can be explained by the widespread diversity evident at a molecular level. Consequently, further stratification of tumors is required; the proposed consensus molecular subtypes consist of a combination of molecular subtyping and specific biomarkers that can provide additional prognostic information above the traditional TNM staging [2]. Further assessments could potentially identify subpopulations of patients that would benefit from additional treatment despite favourable TNM classification. The pertinence of this was clearly demonstrated in this case, where no additional treatment was deemed to be required post operatively based on the initial favourable TNM pathology.
Hypoxia is an alternative biomarker shown to be a potential marker for prognosis in colorectal cancer. Hypoxia inducible factor 1 alpha (HIF1a) induction is established as the key driver allowing cells to adapt to hypoxia as reflected by subsequent downstream expression of genes involved in angiogenesis, glycolysis and cell survival. In colorectal cancer, a large prospective cohort study has evaluated HIF1a expression in relation to prognosis [11]. The study demonstrated that HIF1a expression, as determined by cytoplasmic positivity on immunohistochemistry, was independently associated with poor prognosis [11]. A subsequent meta-analysis of 23 studies also demonstrated a strong association between HIF1a overexpression and increased mortality risk; both overall survival and disease-free survival correlated with HIF1a expression using nuclear or cytoplasmic staining [12]. Despite the evidence of association with prognosis and outcomes, assessment of hypoxia and HIF is not yet included in the routine assessment of colorectal cancer patients and is not considered in the colorectal consensus molecular subtypes. One potential explanation for this is the variability of HIF expression and the reliability of its assessment by immunohistochemistry; not all studies identify a positive correlation, with a recent retrospective analysis of 186 patients demonstrating no significant correlation between HIF1a, CAIX and survival outcomes [13].
An alternative method of assessing hypoxia involves PET imaging using hypoxia selective radiotracers such as [18F ]FMISO or [18F]FAZA. These have the benefit of assessing the entire tumor, being non-invasive, permitting evaluating of hypoxia at multiple time points and distinguishing between acute and chronic hypoxia. There is minimal clinical data in the literature regarding hypoxia PET/CT in colorectal cancer, with the majority of previous studies focusing on H&N, lung, and GBM [3], with only one study demonstrating the feasibility of [18F]FAZA as a hypoxia imaging marker in colorectal cancer [6]. Furthermore, although a correlation of FAZA uptake with IHC has been demonstrated previously in the preclinical setting, it has yet to be convincingly demonstrated in the clinical setting [9, 14]. One study has demonstrated CAIX correlation with FAZA in non-small cell lung cancer (NSCLC) in one case [10]. We add to the literature by presenting the first case of colorectal cancer demonstrating this correlation.
The correlation of FAZA uptake with immunohistochemistry hypoxia markers is a crucial step in tracer validation as a hypoxia biomarker. Mapelli et al. demonstrated a positive correlation in one NSCLC case, but two NSCLC cases without FAZA uptake also demonstrated CAIX positivity [10]. Possible explanations for this include the difference between acute and chronic hypoxia. Given the limited correlations of this in the current literature, further work is required in this area to fully validate FAZA as a reliable hypoxia PET/CT tracer in the clinical setting.
4. Conclusion
Assessment of tumor hypoxia by PET/CT is a promising biomarker; there is a clear correlation between hypoxia PET/CT uptake and poorer prognosis. This case demonstrates how patients might benefit from the recognition of hypoxia in otherwise good prognostic tumors. The hypoxic nature of the patient’s tumor could potentially explain the discrepancy in the original favourable low-risk pathology and the unexpected development of metastases, highlighting the use of PET/CT as a specific tool for the stratification for hypoxia-related treatment modification. This case provides the first clinical correlation of FAZA uptake and CAIX IHC in a colorectal cancer case, a key aspect in FAZA tracer validation. Further research is required to ensure standardisation of the definition of hypoxia, the development of standard imaging protocols, the reproducibility of these imaging biomarkers and their clinical applicability [3].
Author Contributions
Kirsten Laws: Conception of the work, Design of the work, Interpretation of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Graeme Murray: Design of the work, Acquisition of data, Analysis of data, Interpretation of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Keith Kerr: Design of the work, Acquisition of data, Analysis of data, Interpretation of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fergus McKiddie: Design of the work, Acquisition of data, Analysis of data, Interpretation of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sergio Dall’Angelo: Acquisition of data, Analysis of data, Interpretation of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matteo Zanda: Acquisition of data, Analysis of data, Interpretation of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ian Fleming: Conception of the work, Design of the work, Interpretation of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Leslie Samuel: Conception of the work, Design of the work, Interpretation of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Funding for the pilot study was provided by the Colorectal Study Fund, a NHSG endowment fund number: NER 11482.
Ethical Approval
Ethical approval for pilot CRC FAZA PET study was given by North of Scotland REC, reference number: 12/NS/0112.
Consent
Fully informed consent was obtained from the patient to take part in the study. Fully informed consent was also obtained for publication in the form of this case report.
Data Availability
All data used and analysed for the study are available from the corresponding author on reasonable request.
Abbreviations
[18F]FAZA: [18F]Fluoroazomycin Arabinoside
[18F]FMISO: [18F]Fluoromisonidazole
[18F]DG: [18F]Fluorodeoxyglucose
CAIX: Carbonic Anhydrase Nine
CMS: Consensus Molecular Subtypes
GBM: Glioblastoma
H&E: Haematoxylin and Eosin
H&N: Head and Neck Cancers
HIF1a: Hypoxia Inducible Factor 1 alpha
IHC: Immunohistochemistry
MSI: Microsatellite Instability
NSCLC: Non-Small Cell Lung Cancer
PET/CT: Positron Emission Tomography / Computed Tomography
PR: Per Rectum
RCC: Red Cell Concentrates
RML: Right Lung, Middle Lobe
RUL: Right Lung, Upper Lobe
SUV: Standardised Uptake Value
T:B: Tumor to Background ratio
T:M: Tumor to Muscle Ratio
TNM: Tumor Node Metastasis Staging
UGIE: Upper Gastrointestinal Endoscopy
WHO: World Health Organisation
REFERENCES
- Puppa G, Sonzogni A, Colombari R, et al. “TNM staging system of colorectal carcinoma: a critical appraisal of challenging issues.” Arch Pathol Lab Med, vol. 134, no. 6, pp. 837-852, 2010. View at: Publisher Site | PubMed
- Guinney J, Dienstmann R, Wang X, et al. “The consensus molecular subtypes of colorectal cancer.” Nat Med, vol. 21, no. 11, pp. 1350-1356, 2015. View at: Publisher Site | PubMed
- Fleming IN, Manavaki R, Blower PJ, et al. “Imaging tumour hypoxia with positron emission tomography.” Br J Cancer, vol. 112, no. 2, pp. 238-250, 2015. View at: Publisher Site | PubMed
- Bollineni VR, Kerner GS, Pruim J, et al. “PET imaging of tumor hypoxia using 18F-fluoroazomycin arabinoside in stage III-IV non-small cell lung cancer patients.” J Nucl Med, vol. 54, no. 8, pp. 1175-1180, 2013. View at: Publisher Site | PubMed
- Mortensen LS, Johansen J, Kallehauge J, et al. “FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial.” Radiother Oncol, vol. 105, no. 1, pp. 14-20, 2012. View at: Publisher Site | PubMed
- Havelund BM, Holdgaard PC, Rafaelsen SR, et al. “Tumour hypoxia imaging with 18F-fluoroazomycinarabinofuranoside PET/CT in patients with locally advanced rectal cancer.” Nucl Med Commun, vol. 34, no. 2, pp. 155-161, 2013. View at: Publisher Site | PubMed
- Sato J, Kitagawa Y, Yamazaki Y, et al. “18F-fluoromisonidazole PET uptake is correlated with hypoxia-inducible factor-1alpha expression in oral squamous cell carcinoma.” J Nucl Med, vol. 54, no. 7, pp. 1060-1065, 2013. View at: Publisher Site | PubMed
- Norikane T, Yamamoto Y, Maeda Y, et al. “Correlation of (18)F-fluoromisonidazole PET findings with HIF-1alpha and p53 expressions in head and neck cancer: comparison with (18)F-FDG PET.” Nucl Med Commun, vol. 35, no. 1, pp. 30-35, 2014. View at: Publisher Site | PubMed
- de Bruin LB, Bollineni VR, Wachters JE, et al. “Assessment of hypoxic subvolumes in laryngeal cancer with (18)F-fluoroazomycinarabinoside ((18)F-FAZA)-PET/CT scanning and immunohistochemistry.” Radiother Oncol, vol. 117, no. 1, pp. 106-112, 2015. View at: Publisher Site | PubMed
- Mapelli P, Bettinardi V, Fallanca F, et al. “18F-FAZA PET/CT in the Preoperative Evaluation of NSCLC: Comparison with 18F-FDG and Immunohistochemistry.” Curr Radiopharm, vol. 11, no. 1, pp. 50-57, 2018. View at: Publisher Site | PubMed
- Baba Y, Nosho K, Shima K, et al. “HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers.” Am J Pathol, vol. 176, no. 5, pp. 2292-2301, 2010. View at: Publisher Site | PubMed
- Chen Z, He X, Xia W, et al. “Prognostic value and clinicopathological differences of HIFs in colorectal cancer: evidence from meta-analysis.” PLoS One, vol. 8, no. 12, pp. e80337, 2013. View at: Publisher Site | PubMed
- Saka B, Ekinci O, Dursun A, et al. “Clinicopathologic and prognostic significance of immunohistochemical expression of HIF-1alpha, CXCR4 and CA9 in colorectal carcinoma.” Pathol Res Pract, vol. 213, no. 7, pp. 783-792, 2017. View at: Publisher Site | PubMed
- Busk M, Horsman MR, Jakobsen S, et al. “Imaging hypoxia in xenografted and murine tumors with 18F-fluoroazomycin arabinoside: a comparative study involving microPET, autoradiography, PO2-polarography, and fluorescence microscopy.” Int J Radiat Oncol Biol Phys, vol. 70, no. 4, pp. 1202-1212, 2008. View at: Publisher Site | PubMed
