Telomeres are DNA-protein complexes present at the ends of the chromosomes, composed of tandem “TTAGGG” repeats ranging about 15 kilobases in length. Telomere comes from the Greek word telos, meaning “end”, and meros, meaning “part.” Telomere length is maintained by the enzyme “telomerase” – a ribonucleoprotein reverse transcriptase enzyme, expressed in germ cells, stem cells, and regenerating tissues. However, there is insufficient telomerase in somatic cells to indefinitely maintain telomere length, and most tissues have deficient telomerase levels.
Effects of Telomeres with Stress
Telomere length serves as a biological counter, ticking off the passage of time with each cell division, and telomeres are observed to shorten with age in most somatic tissues. Telomere shortening is influenced by recombination, epigenetic regulation, and genetic factors, as well as oxidative stress, and the ability of telomerase to counteract these influences is limited. The shortening of the telomeres (the DNA-protein caps present at the ends of the chromosomes that protect genes from degradation) marks the gradual aging of the immune system. The length of the telomere is a potential marker of cell aging. Telomere length is associated with chronic stressors in adulthood and early adversity. Stress over the lifespan is thought to promote the accelerated aging and illness in early life. Accumulated adverse childhood experiences significantly showed an increased likelihood of having short telomeres later in the life span. This suggests a potential pathway through which childhood experiences have been previously shown to predict adulthood morbidity and mortality.
Telomere length is linked prospectively and cross-sectionally with human diseases in several studies. The cumulative adversity throughout the lifespan has bigger impacts than single events; it is also possible that adversity in childhood has larger effects on later life than adulthood stresses. A current meta-analysis suggests that individuals with observed short leukocyte telomeres are at ∼80% increased risk of concurrent reports of cardiovascular disease and ∼40% increased risk of developing cardiovascular disease in the future. Several studies indicate that short telomeres, including leukocytes and saliva, are related to early mortality. Even though the studies suggest that telomere length plays a role in disease. Reduced telomere length acts as a substitute for cellular aging and is also associated with numerous chronic somatic diseases that are generally considered diseases of aging, including diabetes, cancer, and heart disease. Currently, shorter telomeres have been demonstrated in several psychiatric conditions, mainly depression. Various sustained psychosocial stress in adulthood appears to be associated with shorter telomeres.
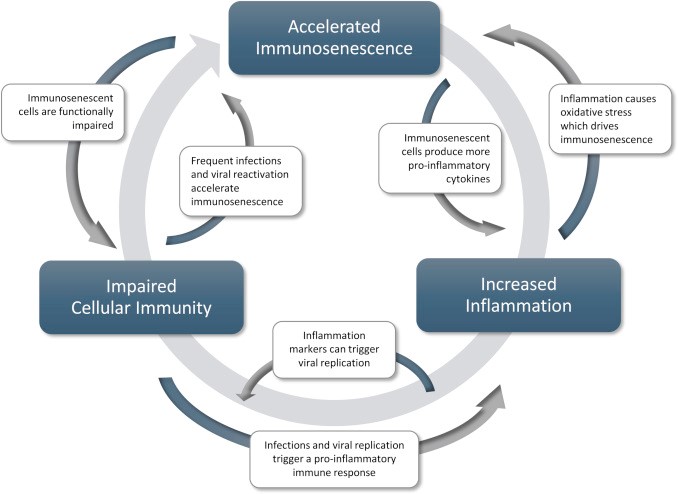
How Telomeres Experience Length Shortening
Telomeres are a protective covering at the end of each strand of DNA that protects the DNA from wear and tear inside the cell. Every time a cell divides, it loses a portion of its telomeres. Enzyme telomerase can replenish the lost telomere, but specific chronic stress and cortisol exposure decrease the supply. When the telomere becomes too diminished, the cell mostly dies or becomes pro-inflammatory.
Other Facts About Telomeres
The complexities of telomere study and its consequences continue to deepen with further research. Telomere length ranges from highly to moderately heritable and estimates from 36% to 84% heritability. Exposure to adversity has the capability to degrade cell function and accelerate the aging of the immune system. Adversity at different time points throughout the lifespan includes prenatal exposure to maternal stress, and repeated experiences of abuse during childhood, followed by adulthood stressors such as exposure to financial and life stressors associated with caregiving or poverty.
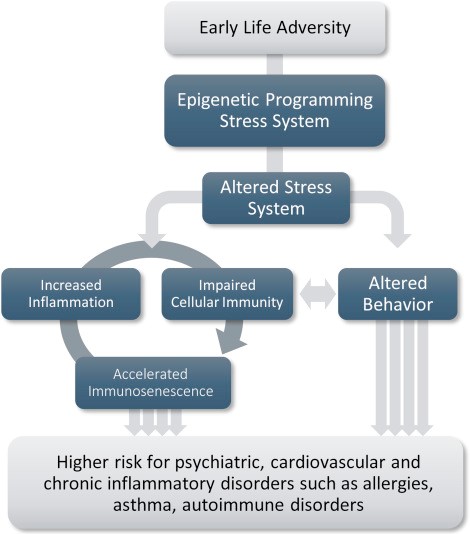
The long-term sequelae of adverse early-life experiences have been a focus in psychiatry, with neurobiological emphasis on physiological systems that are stress-responsive, including the hypothalamic-pituitary-adrenal (HPA) axis and neuroimmune function. Telomere length in proliferative tissues declines with cell replication, whose effects can be accelerated by factors like inflammation, oxidative stress, radiation, and toxins. Recently, an etiological role for early-life stress has been documented for several prevalent somatic conditions, including irritable bowel syndrome, chronic fatigue syndrome, fibromyalgia, obesity, migraine, and chronic pain.
A drawback lies in using telomere length as a clinical measure is the high variability between individuals, present at birth. The length of the telomere is found equal between the sexes at birth. The shortening of telomeres with age occurs more rapidly in males than females, and rates may also differ between ethnic groups. Most clinical studies have utilized telomeres derived from leukocytes since peripheral blood is more easily obtained than most other tissues. A key finding from clinical studies is that alteration of leukocyte telomere dynamics reflects organ dysfunction elsewhere in the body. Telomerase deficiency has been causally linked with the genetic disorder – dyskeratosis congenital, familial idiopathic pulmonary fibrosis, and familial bone marrow failure syndromes.