Nobody knew what killed Robert Rayford, the 15-year-old African American boy when he presented at St Louis city hospital in late 1968. The first known HIV-related death had the medical team draw a blank. For years, the scientific community has been pursuing the holy grail of a complete cure for HIV, the infection linked to the deadly AIDS disease which has claimed 35 million people worldwide since its discovery four decades ago and to this day afflicts nearly 38 million. But the virus’ tricky, constantly mutating nature has so far made it impossible to develop an effective vaccine, even as the constantly improving antiviral drug classes and increasing access to effective HIV prevention, diagnosis, treatment, and care, including for opportunistic infections, has made HIV infection a manageable chronic health condition, enabling people living with HIV to lead long and healthy lives.
A total cure is the need of the hour to restore a normal life expectancy and the immune function, to cut short the cost effect for the individual, family and to his country, to reduce morbidity, mortality, to avoid exposing to drug toxicity, to avoid subsequent loss of functions of vital organs such as kidney, liver, heart, bone marrow, and nervous system, to avoid unnecessary drug interaction, and to avoid acceleration of aging. There are two different visions of a potential HIV cure: treatment-free remission and viral eradication. It is important to conduct research on finding an effective vaccine because:
• The availability of a safe, highly effective, and accessible preventive HIV vaccine would be a valuable complement to other preventive interventions, significantly contributing to the interruption of the chain of transmission of HIV.
• Well-conceived HIV immunization strategies could reach populations where other interventions are not sufficiently effective.
• Research on preventive HIV vaccines is providing new information on the possible use of vaccines as therapeutic interventions, to be used in association with anti-retroviral therapies (ART), which could lead to a lowering in the cost of the treatments and to an increase on their long-term efficacy.
The classical vaccination approach that is successful in the control of other viral diseases – priming the adaptive immunity to recognize the viral envelope proteins – did not work against HIV. Many factors make the development of an HIV vaccine different from other classic vaccines:
• Classic vaccines mimic natural immunity against reinfection as seen in individuals recovered from infection; there are almost no recovered AIDS patients.
• Most vaccines protect against disease, not against infection; HIV infection may remain latent for long periods before causing AIDS.
• Most effective vaccines are whole-killed or live-attenuated organisms; killed HIV-1 does not retain antigenicity and the use of a live retrovirus vaccine raises safety issues.
• The epitopes of the viral envelope are more variable than those of many other viruses. Furthermore, the functionally important epitopes of the gp120 protein are masked by glycosylation, trimerisation and receptor-induced conformational changes making it difficult to block with neutralizing antibodies.
• First, HIV is highly mutable. Because of the virus's ability to rapidly respond to selective pressures imposed by the immune system, the population of virus in an infected individual typically evolves so that it can evade the two major arms of the adaptive immune system; humoral (antibody-mediated) and cellular (mediated by T cells) immunity.
• Second, HIV isolates are themselves highly variable. HIV can be categorized into multiple subtypes with a high degree of genetic divergence. Therefore, the immune responses raised by any vaccine need to be broad enough to account for this variability. Any vaccine that lacks this breadth is unlikely to be effective.
The difficulties in stimulating a reliable antibody response has led to the attempts to develop a vaccine that stimulates a response by cytotoxic T-lymphocytes. Another response to the challenge has been to create a single peptide that contains the least variable components of all the known HIV strains.
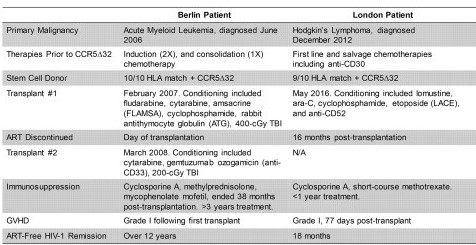
Some promising approaches to achieve an HIV cure include allogeneic hematopoietic stem cell transplantation from a donor homozygous for HIV-resistant CCR5Δ32 mutation, alteration of the host genome using genetic engineering techniques like CRISPR, the “kick and kill” or “shock and kill” method (where immune stimulants shock the latent virus from hidden reservoirs and then attempt to kill reactivated HIV), altering the immune system for better clearance of the HIV-infected targets, and the “hit early and hit hard” method (early and aggressive treatment with ART). Several preventive and therapeutic vaccine candidates are in varying phases of clinical trials (picture below), although some researchers seem to think that the pipeline is not nearly long enough.
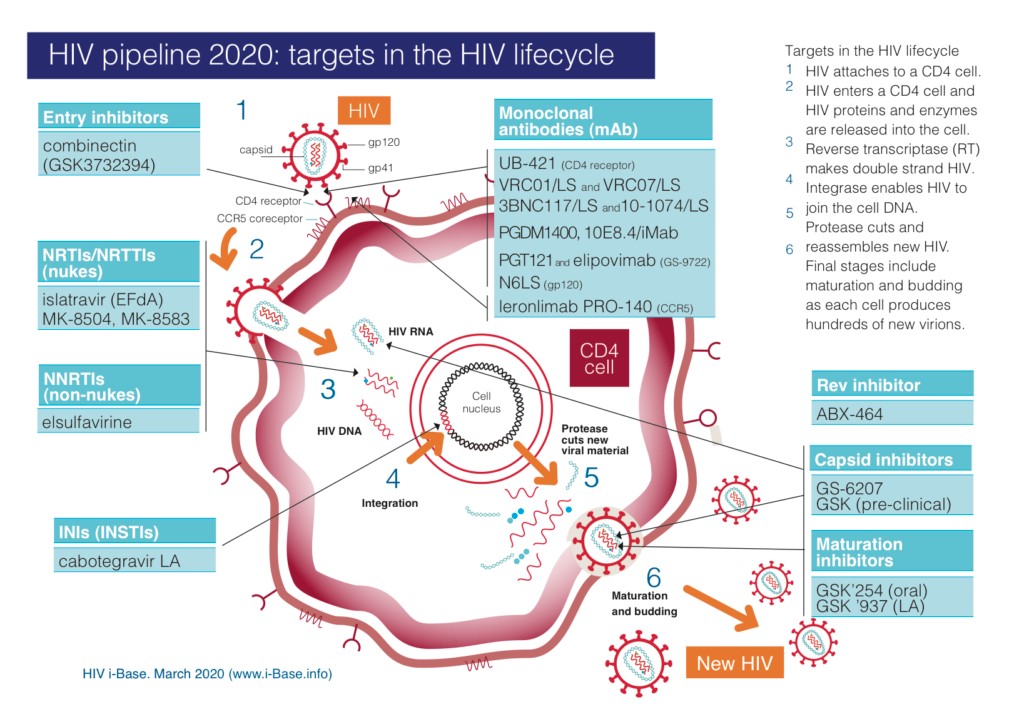
The WHO puts the global investment on HIV vaccine research, including industry and research agencies in industrialized countries at approximately US$ 500 million per year. A very small fraction of that amount is dedicated to the activities aimed at developing vaccines for developing countries. This level of investment is insufficient to carry on the simultaneous development of multiple vaccine products in parallel. The challenges apart, newer reports coming in daily have encouraging outcomes. Moreover, the two cases of the Berlin patient and the London patient (compared in the picture below), both watershed moments in the history of HIV research, indeed offer added hope. We are surely living in an exciting time as scientists currently eyeing a sterilizing cure for HIV disease within another 5-10 years!