Microbes living inside us has turned our view of ourselves inside out. A whole bacterial universe resides in our gut. Human gut, often referred to as the “second brain” not only influences our health but our mind too. This is the only organ possessing its own independent nervous system, an intricate network of 100 million neurons embedded in the gut wall. Numerous experiments in the past few years have confirmed the gut microbiome as a key regulator in development and maintenance of both enteric and central nervous system. Several lines of evidence support the idea of gut microbiome which communicates directly with the gut-brain axis and mediates changes in brain function and behaviour such as depression, anxiety and cognition.
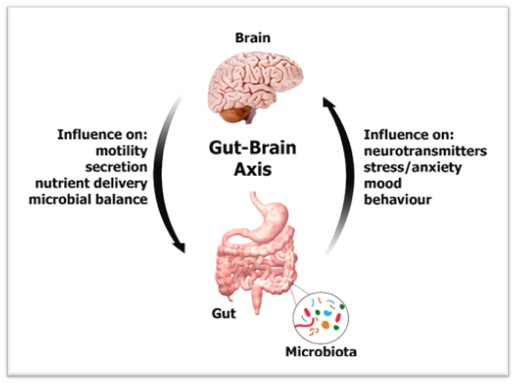
The ongoing research is trying to find out the underlying mechanism and each study turns over another proverbial rock of possibilities. This microbiome-gut-brain axis is believed to modulate various central processes through the vagus nerve as well as to produce microbial metabolites and immune mediators which trigger changes in neurotransmission and behaviour. The stress we experience everyday could deeply affect us and the gut microbiome is playing a crucial role in this.
Evolution of the Gut Microbiome
The gut is sterile prior to birth and it first encounters bacteria when the newborn passes through the birth canal. Various bacteria like Bifidobacterium longum, Lactobacillus helveticus etc are acquired through transmission from mother. As we develop, our gut is exposed to a diversified collection of bacteria, fungi, protists and archaea. These colonize the gastrointestinal tract (GI) tract and are present in a majority of individuals.
Is the way to our brain through our stomach?
Apart from the immunological and metabolic functions, this microbiome is directly linked with mental health. The gut microbiota forms a complex network termed the microbiota-gut-brain axis along with the enteric nervous system. The vagus nerve relays the messages between brain and gut via gut-brain highway. Gut microbiome has bacteria that produce butyrate, a short-chain fatty acid that makes the gut lining less porous. This activity helps prevent metabolites produced by microbes from reaching the brain where they can modify mood and anxiety levels. When the gut microbiota produces enough short-chain fatty acids, it promotes the production of serotonin which regulates mood, happiness and anxiety. Gut microbiome also produces the relaxant Gamma-Aminobutyric Acid (GABA) and its relaxant properties influence the central nervous system.
Depression under the Microscope
The burden of depression is on the rise globally and the pandemic of 2020 has made it worse. There are varieties of treatment approaches for maintaining physical health, but it becomes a bit difficult when our mind is concerned too. A combination of genetic, biological, environmental and psychological factors results in depression which in turn leads to more stress and worsens the affected person’s life situation. Evidence in studies indicate that the microbiome of healthy people appears to differ from those who have a mental illness. An imbalance of the hypothalamic-pituitary-adrenal (HPA) axis results in depression and cytokine activation (interleukins 1 and 6) triggers the release of cortisol, a potent stress hormone. The gut-brain axis, the bidirectional link between the brain and the gut microbiome, is caused by dysregulation of the HPA axis. The bidirectional communication occurs through the signaling pathway which includes hormonal, neural and immune mediators. The unbalanced microbiome may result in a range of non-communicable diseases including obesity, autoimmune disorders, diabetes, autism, multiple sclerosis, epilepsy, Alzheimer’s and Parkinson’s disease and even depression and anxiety.
Stress-induced changes to the microbiome affect both our brain and behaviour. Increased secretion of cortisol, the so-called “stress hormone”, defensive molecules like inflammatory cytokines are produced under stress response and these, in turn, make people more vulnerable to anxiety and depression. Also, stress triggers the risk of developing autoimmune diseases such as by creating microbial dysbiosis in gut. Increasing evidence suggests that the alteration in the gene expression of gut microbiota may stimulate the activities of immune cells in such a way that they attack the body’s own tissue, cells or organs and develop diseases like multiple sclerosis, lupus, rheumatoid arthritis, juvenile diabetes, scleroderma and pulmonary fibrosis. Scientists have found out that depression and anxiety have a direct connection with these autoimmune diseases.
The Promise of Probiotics
Probiotics are good organisms that are intended to have health benefits but in case of depression, the “clinically proven” probiotics sold in markets are how much clinically proved and effective is still a topic of research. Research shows that probiotic gut bacteria can alleviate depression and anxiety. Probiotics can be used to treat anxiety or depression but at this point, there are no compelling data that suggest a true benefit. Probiotics may reduce the production of inflammatory cytokines, or they may enable the action of tryptophan which in turn stimulates serotonin secretion. There are varying classifications of depression and every person’s microbiome is different. Therefore, one standardized treatment will not be effective for others and the growing demand for personalized probiotics could be expensive as well. Thus, more research should be needed to identify the best dose and strain to combat depression.
Towards a Psychobiotic Revolution
Psychobiotics confer mental health benefits by altering the gut microbiome. Any substance that exerts a microbiome-mediated psychological effect is potentially a psychobiotic. The microbiome is sensitive to food and exercise and both of which have mood-boosting benefits. The alteration of the gut microbiome has the potential to control our health and well-being. This could be a completely new way of approaching mental health challenges-a psychobiotic revolution. As John Cryan said, “a state of gut will markedly affect your state of mind, so let food (for your microbes) be thy medicine”.
We are still in the infancy stage of this research and a better understanding of this complex relationship could one day give rise to individualized gut microbe treatments for autoimmune conditions that are sensitive to stress. Further in-depth mechanistic studies on microbiota-autoimmunity interplay are urgently needed and underway to explore novel and precise diagnostic biomarkers and develop disease and patient-tailored therapeutic strategies.