Received: Fri 07, Aug 2020
Accepted: Mon 24, Aug 2020
Abstract
Treatment with 5-FU cream, an antimitotic agent, is primarily used for the treatment of superficial keratinocytic lesions. This treatment modality has the potential to cause severe localized inflammation with subsequent erythema, pain and crusted erosions. Cutaneous mucormycosis is an opportunistic emerging fungal infection. It is rapidly progressive and affects immunosuppressed or poorly controlled diabetic patients primarily by direct inoculation or secondarily by dissemination. The clinical presentation can be challenging due to its initial nonspecific features and therefore leading to a delay in the diagnosis. Herein, we report a case of mucormycosis of the scalp following treatment with topical 5-FU.
1. Introduction
Cutaneous mucormycosis is a very fatal, rapidly progressing opportunistic fungal infection. It may be acquired primarily by direct inoculation through inhalation or through the skin as a port of entry. Treatment with fluorouracil (5-FU) cream for actinic keratosis or other superficial keratinocytic carcinomas can cause a severe localized therapeutic inflammatory response. We report a case of S. vasiformis related necrotic infection of the scalp following treatment with topical 5-FU.
2. Case Report
An 84-year-old male with a medical background of ischemic heart disease, hypertension, type-2 diabetes mellitus and chronic renal failure was referred to the emergency department (ED) due to a tender necrotic lesion on the scalp accompanied by erythema, burning sensation and face swelling that evolved over two weeks. Two months prior to his presentation, the patient underwent an excisional biopsy of a cutaneous lesion from the scalp, which revealed well-differentiated squamous cell carcinoma (SCC) involving one lateral margin. After the resection, topical 5-FU cream was initiated along with aflumycin cream (prednisolone 0.5%, gentamicin sulphate 0.16%). During treatment, a necrotic lesion appeared on the resected scalp surface. The lesion was inspected by several physicians, and the patient was treated with antibiotics for cellulitis (five days of cefazolin and one day of ciprofloxacin). Due to lack of improvement, he was referred to the ED.
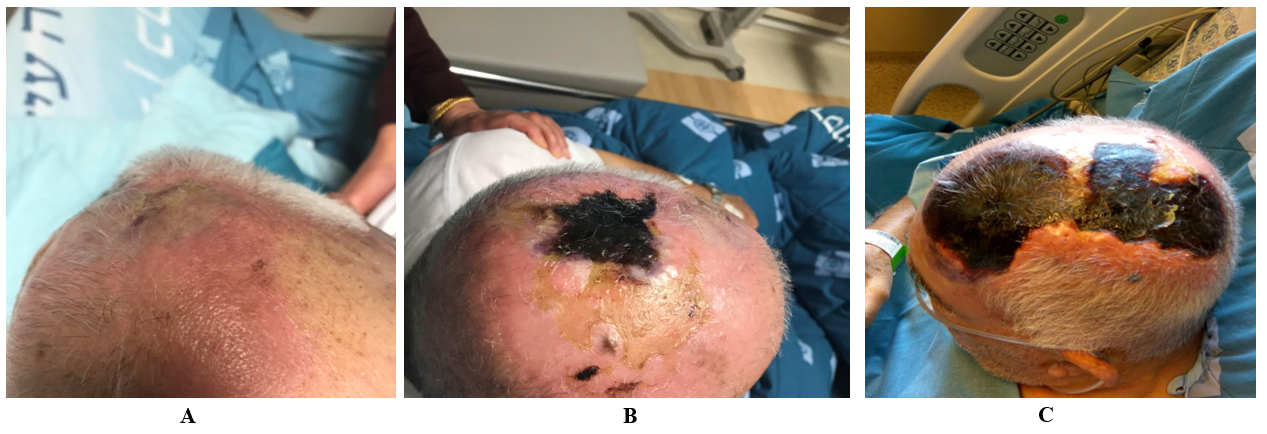
Upon arrival, his vital signs were normal (heart rate 72 beats/min, Blood pressure 130/70 mmHg, fever 37.2°, Saturation 96% RA). Physical examination revealed a 3X3 cm necrotic lesion on the mid-scalp region surrounded by inflammation with local heat and tenderness covering 30% of the scalp (Figure 1A, 1B). Laboratory tests showed acute on chronic renal failure (plasma creatinine 243 \μ mol/L , whereas his baseline value was 140 \μ mol/L), leukocytosis 14.1 X 109/L with neutrophilia 87% and an elevated C-reactive protein of 17.7mg/dl (normal range 0-0.5mg/dl). Pulmonary congestion was noticed in chest X-ray. The patient was admitted to the internal medicine department with a diagnosis of topical 5-FU related contact dermatitis with suspected secondary cellulitis. Intravenous cefazolin was commenced and later substituted with piperacillin-tazobactam and vancomycin for lack of clinical progress.
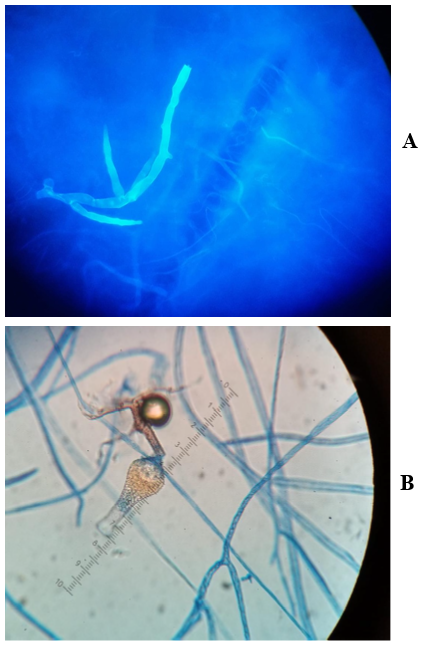
Despite treatment, the necrotic lesion was progressing rapidly in size (Figure 1C). At this point of time, a biopsy of the lesion was performed. Both histopathology and calcofluor-white-stain for fungi revealed broad non-septate hyphae (Figure 2A). The culture growth was identified morphologically as Saksenaea vasiformis, which was also confirmed by real time PCR. The patient was immediately treated with liposomal amphotericin-B and underwent a total removal of scalp, followed by two additional debridements, with no evidence of skull involvement. Due to somnolence, hypoxia, and acute kidney injury, he was mechanically ventilated and dialysis was commenced. Unfortunately, he developed an abdominal swelling and underwent a CT scan revealing pneumatosis intestinali. In light of his general condition, it was decided to keep the patient on supportive care, and 27 days after admission, the patient succumbed.
3. Discussion
Mucormycosis is an uncommon opportunistic fungal infection. It presents very rapidly and aggressively and is usually fulminant [1]. Primary infections of mucormycosis arise from direct inoculation, and secondary infections are caused by dissemination [2]. Although rhino-orbito-cerebral involvement is the most common presentation of secondary infections, pulmonary, gastrointestinal as well as cutaneous involvement can occur as well [2, 3]. Saksenaea vasiformis, a member of the order Mucorales, is mainly isolated from soil [4]. Unlike other members of the group, it mainly affects cutaneous and subcutaneous tissues following trauma, burns, or lacerations, usually following health care-associated exposures [5]. So far, only 40 cases of clinical S. vasiformis infections were reported.
Cutaneous lesions of S. vasiformis initially present as erythematous-purple papules or nodules evolving to necrosis or eschar following angio-invasion [6, 7]. Ulcerations may also occur [8]. The lesions are sometimes misdiagnosed as cellulitis, allergic contact dermatitis or other necrotizing soft tissue infections [9]. The rapid and aggressive progression requires prompt surgical and antifungal treatment [1]. S. vasiformis does not easily sporulate in routine mycological media [4] and requires nutrient-deficient media for growth [10]. Thus, difficulty in diagnosis further delays appropriate treatment and contributes to the high mortality rate [11].
Topical 5-FU (available as 0.5%, 1%, and 5% cream) is a pyrimidine analog that interferes with DNA synthesis by blocking the thymidylate synthetase conversion of deoxyuridylic acid to thymidylic acid. It is primarily used in the treatment of actinic keratosis, bowen disease, superficial basal cell carcinoma and warts. Common side effects of topical 5-FU include erythema, dryness, and burning [12]. Severe, although uncommon adverse reactions from topical 5-FU include allergic contact dermatitis [13].
In this case, the delay in diagnosing cutaneous mucormycosis was related to the misassumption of 5-FU as a cause of necrosis. Mucormycosis was considered only when the lesion markedly increased in size. Nevertheless, it is possible that fungal penetration and progression secondary to the local trauma after SCC resection was facilitated when 5-FU was applied due to its immunosuppressive properties.
4. Conclusion
This case illustrates the importance of keeping a high level of suspicion of mucormycosis when cutaneous necrotic lesions are encountered, especially in the context of local trauma. The immunosuppressive effects of 5-FU might have facilitated the progression of the infection in this case.
Conflicts of Interest
None.
Funding
None.
REFERENCES
- Dounia Bitar, Dieter Van Cauteren, Fanny Lanternier, et al. “Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006.” Emerg Infect Dis, vol. 15, no. 9, pp. 1395-1401, 2009. View at: Publisher Site
- Ana Daniela Castrejón Pérez, Esperanza C Welsh,1 Ivett Miranda, et al. “Cutaneous mucormycosis.” An Bras Dermatol, vol. 92, no. 3, pp. 304-311, 2017. View at: Publisher Site
- Mohammad Simbli, Fayaz Hakim, Mohammad Koudieh, et al. “Nosocomial post-traumatic cutaneous mucormycosis: a systematic review.” Scand J Infect Dis, vol. 40, no. 6-7, pp. 577-582, 2008. View at: Publisher Site | PubMed
- Liyanage Shamithra Madhumali Sigera, Kavinga Kalhari Kobawaka Gamage, Melani Naamal Jayawardena, et al. “Cutaneous mucormycosis caused by Saksenaea vasiformis in a patient with systemic lupus erythematosus.” Clin Case Rep, vol. 6, no. 9, pp. 1730-1734, 2018. View at: Publisher Site | PubMed
- I J Padmaja, T V Ramani, S Kalyani “Cutaneous zygomycosis: necrotising fascitis due to Saksenaea vasiformis.” Indian J Med Microbiol, vol. 24, no. 1, pp. 58-60, 2006. View at: Publisher Site | PubMed
- J Torell, B H Cooper, N G Helgeson “Disseminated Saksenaea vasiformis infection.” Am J Clin Pathol, vol. 76, no. 1, pp. 116-121, 1981. View at: Publisher Site | PubMed
- Gomes Marisa ZR, Russell E Lewis, Dimitrios P Kontoyiannis “Mucormycosis caused by unusual mucormycetes, non-Rhizopus,-Mucor, and-Lichtheimia species.” Clin Microbiol Rev, vol. 24, no. 2, pp. 411-445, 2011.
- Brad Spellberg, John Edwards Jr, Ashraf Ibrahim “Novel perspectives on mucormycosis: pathophysiology, presentation, and management.” Clin Microbiol Rev, vol. 18, no. 3, pp. 556-569, 2005. View at: Publisher Site | PubMed
- Anna K Steve, Valerie A Hurdle, Jevon Y Brown “Orbitomaxillofacial Mucormycosis Requiring Complex Multifactorial Management.” Plastic Reconstr Surg Glob Open, vol. 6, no. 10, pp. e1927, 2018. View at: Publisher Site | PubMed
- A A Padhye, L Ajello “Simple method of inducing sporulation by Apophysomyces elegans and Saksenaea vasiformis.” J Clin Microbiol, vol. 26, no. 9, pp. 1861-1863, 1988. View at: Publisher Site | PubMed
- A Skiada, C Lass Floerl, N Klimko, et al. “Challenges in the diagnosis and treatment of mucormycosis.” Medl Mycol, vol. 56, no. 1, pp. 93-101, 2018. View at: Publisher Site | PubMed
- Komal Chughtai, Rahul Gupta, Sunil Upadhaya, et al. “Topical 5-Fluorouracil associated skin reaction.” Oxf Med Case Reports, vol. 2017, no. 8, pp. omx043, 2017. View at: Publisher Site
- B U G A Meijer, F B de Waard van der Spek “Allergic contact dermatitis because of topical use of 5-fluorouracil (Efudix cream).” Contact Dermatitis, vol. 57, no. 1, pp. 58-60, 2007. View at: Publisher Site | PubMed
