Neurodevelopmental and neurodegenerative disorders are the most significant public health concerns, as the challenge is faced due to lack of disease-modifying treatments. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) or CRISPR-associated protein 9 (Cas9) system is a revolutionary, comprehensive tool that is accelerating biomedical research in the field of genome editing tool and enabling targeted genetic interrogation in any organism and cell type. The repair mechanisms are generally utilized in knock out or introduce selected genes. Genome editing refers to procedure of making accurate and permanent changes in the genetic code of cells, tissues, and whole organisms. Gene therapy with CRISPR-Cas is a highly potential therapeutic strategy for treating genetic neurological diseases, including the spinocerebellar ataxias, Huntington disease, amyotrophic lateral sclerosis, and fragile X syndrome.
Mechanism of CRISPR-Cas
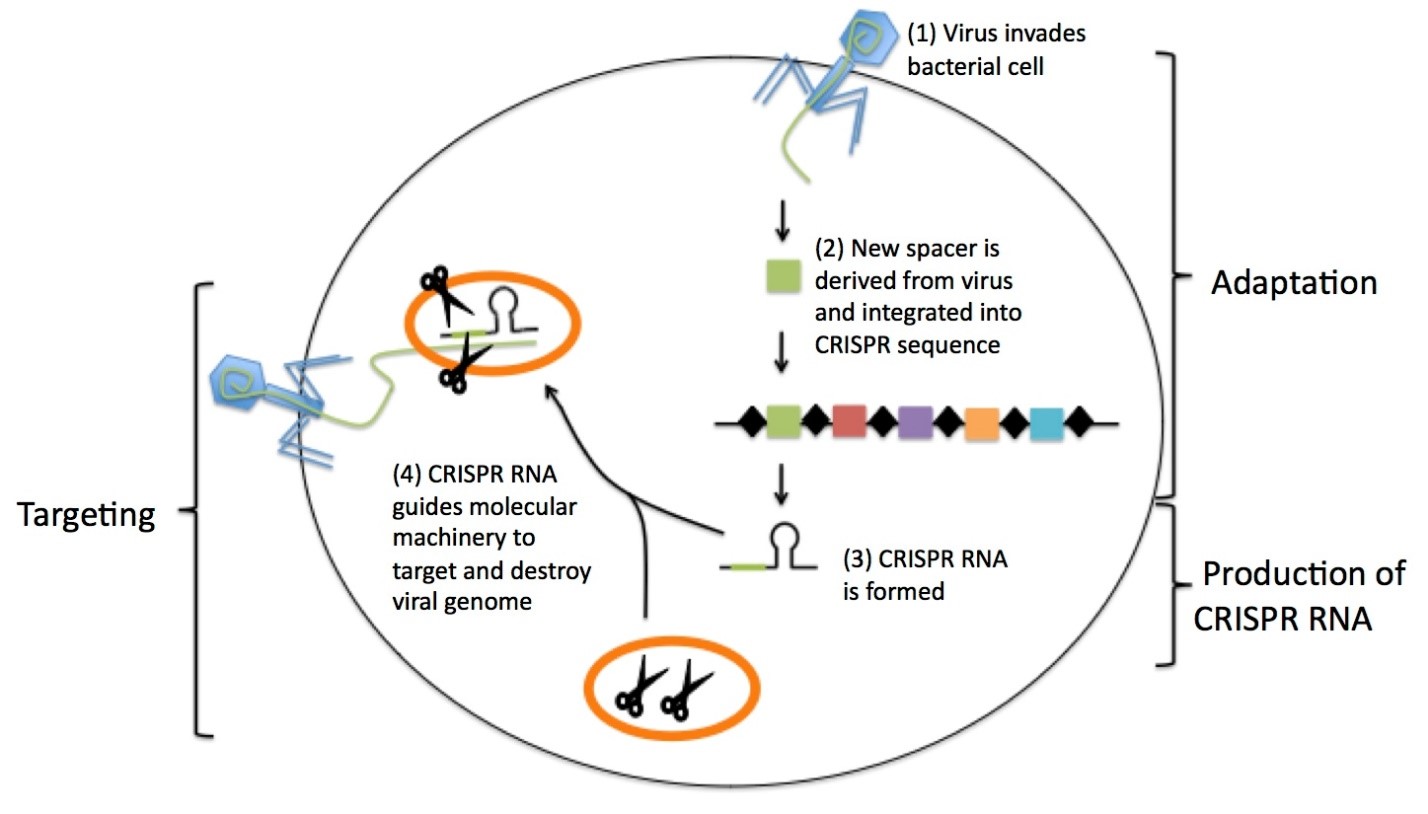
CRISPRs are the regions present in the bacterial genome that acts as the defense against invading viruses. The regions are composed of short DNA repeats and spacer fragments. When a foreign particle such as virus infects a bacterium, a new spacer derived from the virus is incorporated amongst existing spacers. The CRISPR sequence gets transcribed and processed to generate short CRISPR RNA molecules. The CRISPR RNA guides bacterial molecular machinery to a matching target sequence in the invading virus. The molecular machinery cuts and destroys the invading viral genome. Mainly Cas9 protein and guide RNA of CRISPR interact to form a complex that in turn, can identify target sequences with high selectivity and specificity both in natural and in artificial CRISPR/Cas systems. This CRISPR-Cas9 tool uses a ribonucleic acid (RNA)-guided deoxyribonucleic acid (DNA) endonuclease, Cas9, that induces double-strand breaks (DSBs) in the target sites. These double stranded breaks are repaired by various cellular DNA repair mechanisms leading to modification at the target sites. The CRISPR-Cas system act in a sequence-specific fashion by recognizing and cleaving foreign DNA or RNA. CRISPR sequences are vital element of the immune systems. CRISPR-Cas9 technique is a favourable tool in genetic editing due of its high degree of accuracy and flexibility in cutting and pasting DNA.
This defense mechanism can be broadly divided into:
1. Adaptation/spacer acquisition: In the first phase, a distinct sequence of the invading MGE – a protospacer, gets incorporated into the CRISPR array yielding a new spacer. Cas1 and Cas2 are the 2 proteins that is ubiquitously involved in the spacer acquisition process because they can be seen in almost all CRISPR-Cas types.
2. crRNA biogenesis/ expression and processing of CRISPR: The CRISPR array gets transcribed into a precursor crRNA (pre-crRNA) which is further processed into mature guide crRNAs containing the memorized sequences of invaders.
3. Target interference: Mature crRNAs are used as guides to specifically interfere with the invading viral nucleic acid materials. Since crRNA sequences are copied from viral DNA sequences, they act as good guides as they are exact matches to the viral genome.
How CRISPR is Helpful in Neurology?
The CRISPR-Cas9 based genome editing is a simple, advanced, programmable, highly precise, and affordable endonuclease based powerful tool to interrogate and manipulate gene expressions. The discovery of CRISPR has been timed perfectly with the recent neuroscience research boom. Over the past few years, scientists have been using gene sequencing to uncover genes important in brain development and in investigating neurological diseases, including Alzheimer’s disease and schizophrenia. To discover the cause of gene mutation, thousands of people were required previously to be sequenced before scientists could figure out which genetic differences might be linked to the disease. Currently, there are several clues to the genes that might affect a number of disorders like OCD, autism, and major depression. CRISPR came out as the perfect technology to solve this major problem in neurology. The CRISPR-Cas9 system has been adapted to eukaryotic cells, by cultivating the codon structure and developing a linkage loop between the 2 functional ends of the RNA to create a single-guide RNA (sgRNA) molecule.
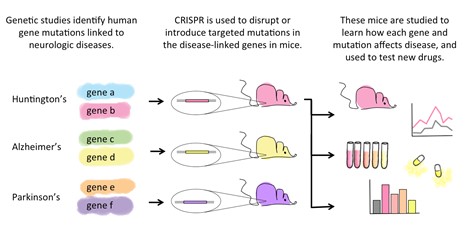
Several neurodevelopmental disorders that are thought to evolve from mutations from a single gene. Since the brain is the yield of millions of connections between neurons, it is crucial to observe the genetic changes in the actual animal brain and find out the potential dugs for the mutated diseases. With the advances of CRISPR-Cas9, targeted manipulation of a variety of regulatory factors, non-coding RNAs, and non-protein-coding sequences can investigate the role of these pathways in neuropsychiatric conditions. Psychiatric and various neurological disorders frequently involve pathogenic alterations in gene-regulatory and epigenetic processes. CRISPR-Cas9-mediated gene-editing can introduce protein tags to target loci, facilitating biochemical and epigenetic studies of endogenous gene products. CRISPR-Cas9 can also be utilized to probe gene-regulatory pathways in hiPSCs models of neuropsychiatric diseases. The trial methods with CRISPR-Cas9 technology will establish itself in the routine clinic, providing an opportunity to save lives.
Limitations
1. The targets used to modify may have resemblance (similarity) in sequences with other parts of the genome that may lead to unintended cuts in the host genome. Thus, undesired mutations are generated.
2. In vivo delivery into the host cell.
3. Setback lies in allele-specific gene correction.
4. The presence of previous antibodies against Cas9 (a protein) or immune response to CRISPR-Cas9 is a significant hurdle.

