Received: Thu 20, Aug 2020
Accepted: Mon 07, Sep 2020
Abstract
This is a description of a novel combination of chromogenic multiplex immunohistochemistry, digital pathology, computer-aided cell detection and topographical analysis of tumor tissue to allow a detailed study of the immune infiltrate. This is applied to a rare clinical case, where a tumor sample is available from an infant with metastatic neuroblastoma at the point of spontaneous regression. This allowed detailed analysis of the immune infiltrate, including spatial distribution and phenotype of lymphoid and myeloid populations, with a distinction between heterogeneous areas within the intra- and extra- tumoral immune microenvironments. The mechanism of spontaneous regression in congenital neuroblastoma is poorly understood, but the data obtained suggested an immune-mediated phenomenon, characterised by an adaptive T cell driven response with a significant delayed-type hypersensitivity (granulomatous) component.
1. Case Presentation
A male infant was delivered at term, having had an adrenal lesion identified on a 32-week antenatal scan. An ultrasound scan (USS) performed on day 2 of life showed a 30x35x40 mm well-circumscribed calcified lesion above the kidney. This was confirmed on subsequent MRI scans. Full blood count, renal and liver function tests were normal. The LDH was 524 U/l (normal 225-425) and urinary catecholamines were elevated (HMMA / creatinine 29 μmol/mmol (normal 0-11), HVA / creatinine 52 μmol/mmol (normal 0-20)). 123-I mIBG imaging showed an area of bright focal uptake in the left adrenal, consistent with localised congenital neuroblastoma. A ‘watch and wait’ strategy was initially followed; the infant remained clinically well with normal growth and development. Surveillance USS showed stable disease until 4 months of age, at which point multiple new small hypoechoic regions within the liver, measuring up to 10 mm in maximum dimensions, were noted. A 123-I mIBG scan at this point showed persistent foci of activity in the area of the primary tumor, but also new activity in the left proximal femur and liver.
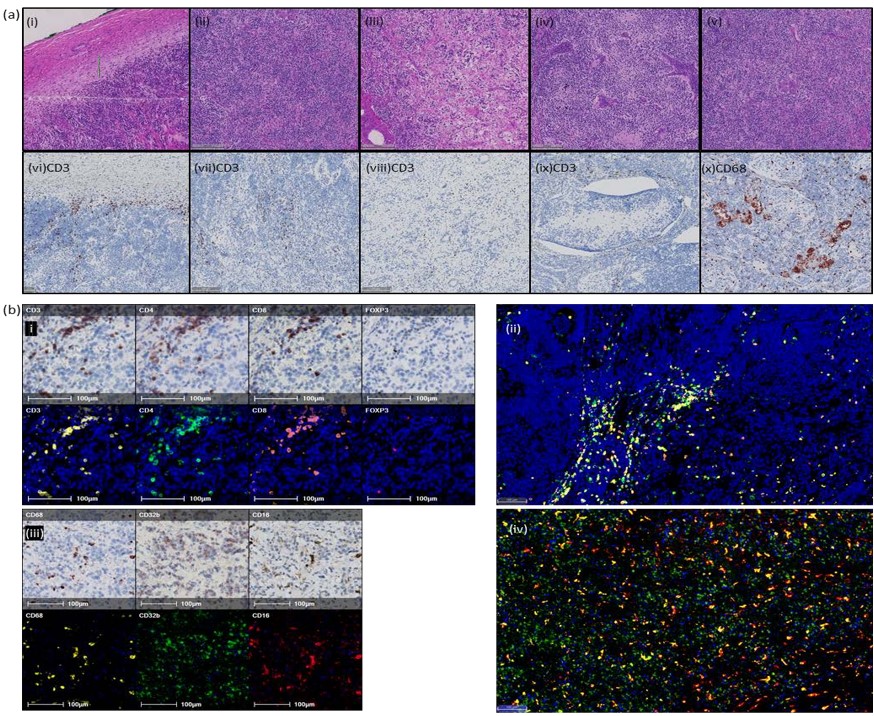
Surgical resection of the primary tumor and a single associated lymph node was performed to obtain the tumor for cytogenetics. Microscopically, the tumor was well-circumscribed and consisted of an undifferentiated neuroblastoma with an intermediate Mitotic Karyorrhectic Index (2.11%) [1, 2]. Immunohistochemical staining was strongly positive for NB84 and CD56, with relatively scanty S100 positive stroma. Cytogenetics showed no evidence of MYC-N amplification and no segmental chromosomal abnormalities. Whole chromosome loss of chromosome 3, 4, 9 and 11, and gain of 17, was seen. The lymph node showed infiltration by neuroblastoma. The tumor contained a marked inflammatory cell infiltrate, with immune cells unevenly distributed throughout the tumor. T cells showed accentuation at the tumor capsule and in cellular areas of the tumor, with a less brisk infiltrate in neuropil rich, hypocellular, areas.
Multiplex chromogenic immunohistochemistry was used to assess the phenotype of the lymphoid and myeloid immune cells. The infiltrate consisted of both CD4 and CD8 positive T cells, with a small component of Regulatory T cells (CD3+.CD4+, FOXP3+). Focally, the lymphocyte infiltrate was arranged in aggregates of mixed populations, which resembled tertiary lymphoid structures, suggesting an in-situ immune response. Pro- inflammatory, activated, macrophages (CD68+, CD16+) and alternatively activated macrophages (CD68+, CD32b+) were present throughout the tumor. In addition, there were areas of granulomatous inflammation, characterised by multinucleated giant cells. These were CD68+ and predominantly CD16+, CD32b-. Interestingly, they were also CD4+, which is known to be expressed by a subpopulation of macrophages, although the significance of this is unknown.
Post-operative imaging was performed two months post resection, and the liver lesions were resolved spontaneously; five months post-surgery, the activity of the proximal left femur lesion had significantly regressed. Eleven months after the operation, the left femur lesion had been totally resolved and the catecholamine in the urine level normalised. The child did not receive any chemotherapy, is now 5 years and 9 months old and has no evidence of disease.
2. Methods
In order to investigate and characterise the immune response, a representative block was chosen, and two serial sections were cut for chromogenic multiplex immunohistochemistry (IHC) [3]. The first section was sequentially stained for T cell markers CD3, CD4, CD8 and FOXP3. The second section was stained for macrophage markers CD68, CD32b and CD16. It was noted that the neuroblastoma (NB) cells express CD32b by IHC, a phenomenon previously reported in NB and other neuroectodermal malignancies [4, 5]. Microanatomical structures and areas of morphological heterogeneity were identified on H+E and IHC brightfield images by a qualified histopathologist (Figure 1A).
Following each round of staining, the slides were imaged and digitised using a Zeiss Axio Scan. QuPath [6] slide analysis software was used to identify and export areas where IHC staining was optimal. Image J (register virtual stack slides plugin) was used to register each image; these were subjected to colour deconvolution to isolate haematoxylin and the AEC chromogen. The images were inverted and converted to 8-bit monochrome images. The registered images were imported to HALOTM digital pathology software [7], and the channels fused to create a composite false-fluorescent image (Figure 1B). The microanatomical structures and areas of morphological heterogeneity were transferred from the registered brightfield images. HALO’s Automated cell detection and classification algorithms were used to identify and quantify immune cells (Figures 2A & 2B).
3. Results
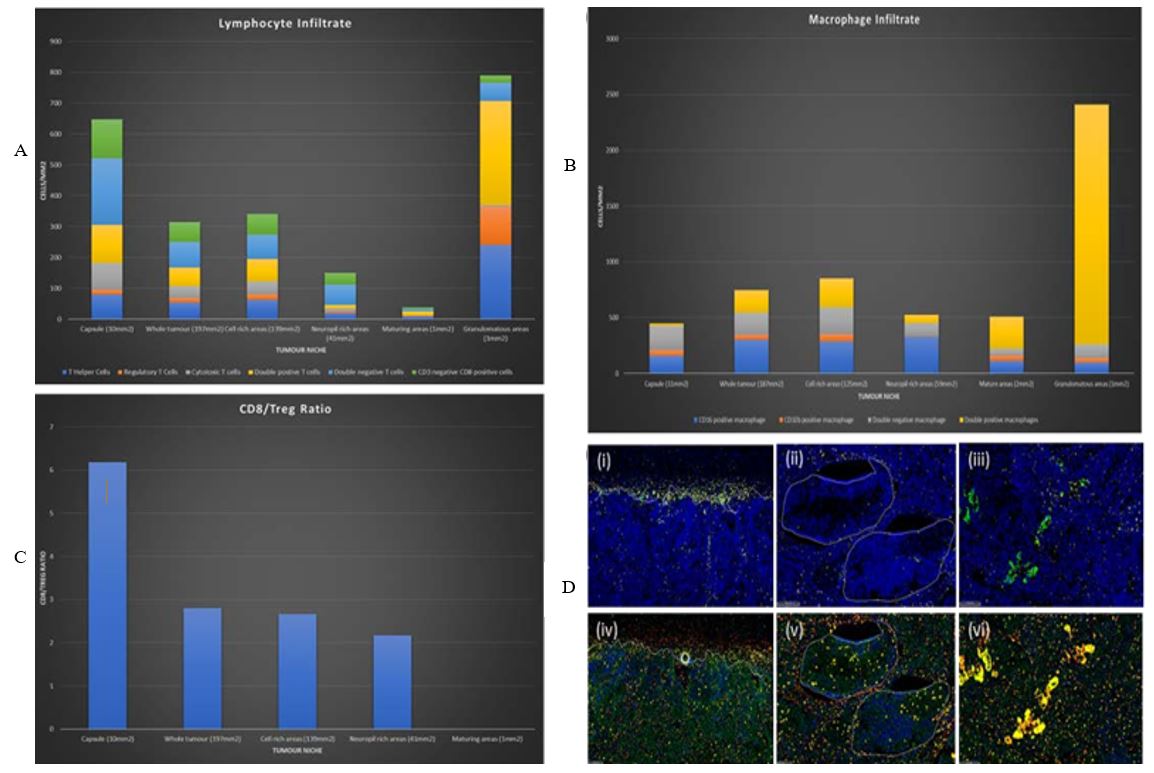
Automated analysis found a brisk immune infiltrate throughout the tumor. Of note, it is overall pro-inflammatory with a high CD8:Treg ratio (>6 in capsule and >2.5 in stroma) (Figure 2C) and an increased density of CD16+ macrophages compared to CD32b+ macrophages (Figure 2B) (CD16+:CD32b+ ratio >3 in capsule and >6 in stroma). The highest immune cell density was around areas of granulomatous inflammation where the cell detection algorithms struggled to resolve individual cells. The density and phenotype of the immune infiltrate varied in line with the tumor heterogeneity. Excluding granulomas, the capsule had the highest density of lymphoid infiltrate and the greatest CD8: Treg ratio, followed by cellular areas of tumor and neuropil rich areas. Mature areas were almost devoid of lymphocyte infiltrate, which is apparent in (Figure 2D).
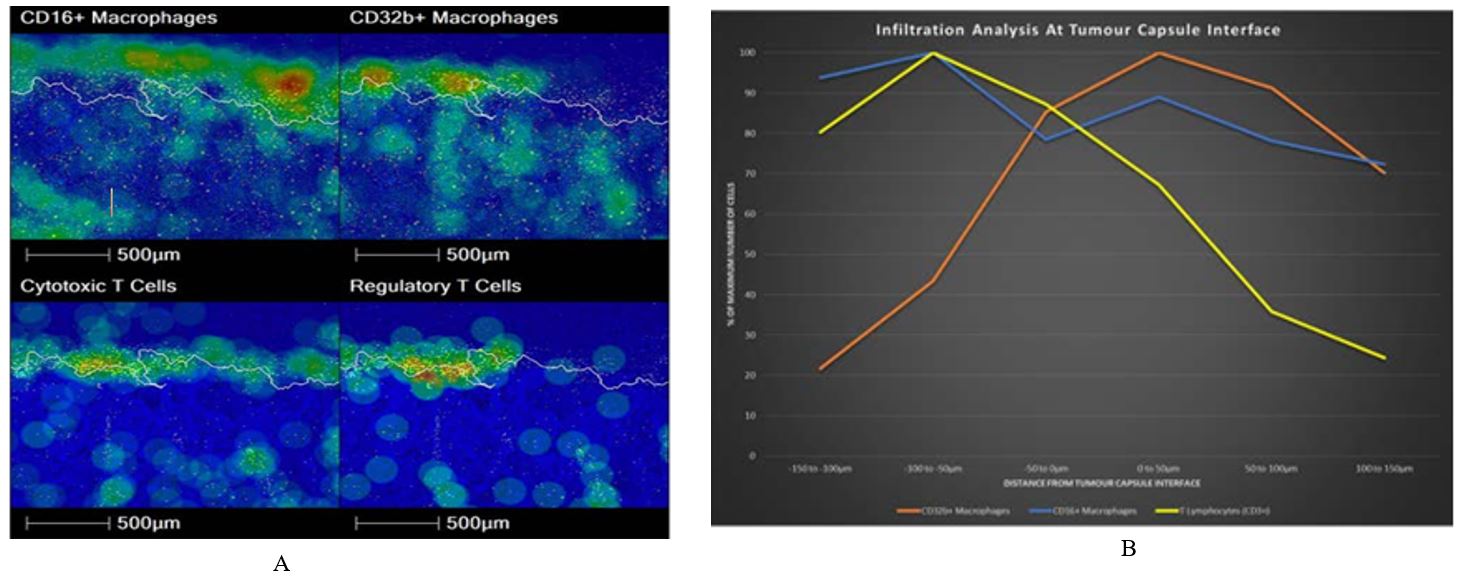
Density heat map and infiltration analysis was done at the tumor capsule, comparing CD16+ macrophages with CD32b+ macrophages and CD8+ T cells with Tregs (Figures 3A & 3B). Figure 3A heat map shows reciprocal localisation of CD16+ and CD32b+ macrophages in the tumor capsule. In addition, regulatory T cells are most dense inside the interface, whilst Cytotoxic T cells are most dense along the interface. Regulatory T cells are less concentrated in areas with a dense CD16+ macrophage population. Figure 3B infiltration analysis shows the maximum density of CD16+ macrophages lies outside of the interface, whereas the maximum density of CD32b+ macrophages is found inside the interface. In addition, Figure 3B shows that CD3+ T cell density decreases moving into the tumor. However, density increases again deeper within the tumor, reflecting a patchy distribution of inflammation (data not shown). Our analysis detected significant populations of double positive and double negative T cells, as well as CD3 null, CD8 positive cells. To investigate this further we closely examined the IHC sections (Figure 4).
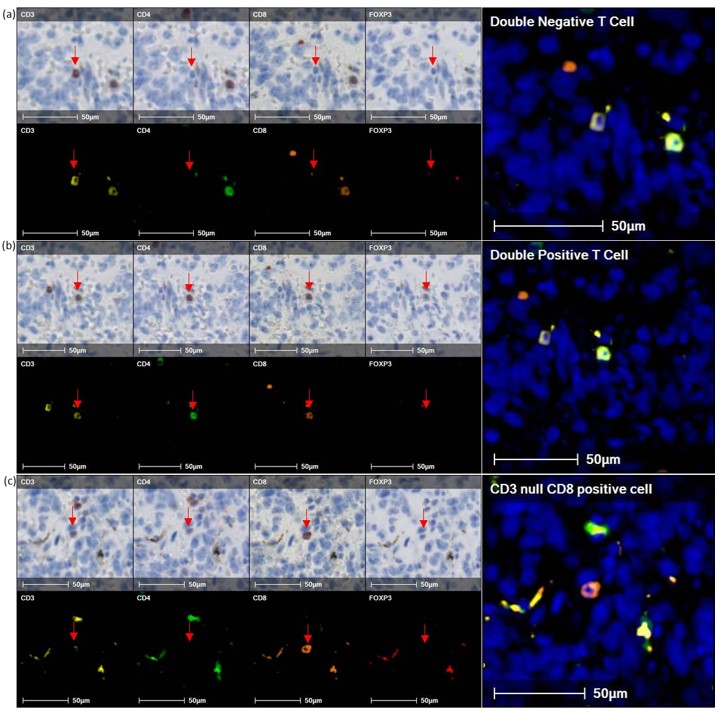
Although staining is often weak for these populations, the presence of nearby internal controls confirms that these phenotypes are not artifacts. This analysis represents a snapshot of an ongoing immune response in which receptors are dynamically regulated. Transient up- and down-regulation of T cell receptor components during activation or senescence is well described and may account for these detections. Alternatively, these non-classical populations may be more abundant in the systemic neonatal immune system or tumor microenvironment. It is likely that CD3 null, CD8 positive cells are NK cells, rather than a T cell population. These observations require further investigation.
4. Discussion
Molecular histopathology is undergoing rapid changes due to simultaneous advances in multiplexing techniques, whole slide imaging and artificial intelligence applied to image analysis. Integrating these technologies is key to understanding the tumor-immune response. This is now a key step in stratifying patients for prognosis and predicting response to therapy. Understanding the complexity of the immune response, in relation to the architecture and heterogeneity of the tumor, is important for optimising and designing immune-based therapies.
Fluorescent multiplexing technology requires specialised and expensive equipment. We have used chromogenic immunohistochemistry, which is available in most conventional research and clinical laboratories. In addition, QuPath and Image J are open-source image analysis platforms. Although HALOTM was used for creating the composite fluorescent image and cell detection, this can also be done using QuPath and Image J. Infiltration analysis and hotspot analysis are currently both limited to the propriety HALOTM software.
Regression of congenital localised or 4S NB is common without chemotherapy [8]; however, regression in metastatic NB is difficult to assess as the majority of cases will receive chemotherapy. In this case, tumor progression, including new metastatic lesions, led to excision biopsy of the primary tumor to obtain diagnostic tissue, including cytogenetics. This gave a unique opportunity to learn about the immune response in spontaneously regressing NB. FFPE tissue normally yields limited information at the single cell level due to the restricted number of markers that can be used in classical IHC. However, combining multiplex analysis with morphometric analysis has allowed us to interrogate this tumor to give important insights into the successful, spontaneous, anti-tumor immune response and how it varies across the tumor.
A number of mechanisms for spontaneous regression of NB have been postulated; these include neurotrophin deprivation, loss of telomerase activity, alternations in epigenetic regulation, or humoral/cellular immunity [8-10]. Classically, NB is considered an immunologically cold tumor. However, this regressing neuroblastoma has a brisk, pro-inflammatory, immune cell infiltrate throughout the tumor, which was intensified at the capsule. Of note, there were no well-developed tertiary lymphoid structures within the tumor itself (a phenomenon we have previously seen following chemoradiotherapy), suggesting that the immune response was evolving extrinsic to the tumor. It is of interest that the myeloid immune component becomes less pro-inflammatory across the capsule and that hotspots of pro- and anti-inflammatory macrophages show reciprocal localisation, indicating there is a complexity to the immune environment throughout the tumor.
Our analysis also studied how the immune infiltrate varied according to tumor’s morphological heterogeneity. Interestingly, lymphocytes were almost absent in areas that had undergone maturation. This could be of clinical significance, as maturation is a feature, and indeed an intention, of conventional chemoradiotherapy and retinoic acid treatment. This may impinge on the success of subsequent immunotherapies, of which monoclonal antibody therapy is now standard. It remains to be investigated whether this immune cell exclusion is due to signals released by mature neuroblastic tissue or geographical exclusion by virtue of larger cell bodies occupying the tissue space and preventing immune cell ingress.
CD4+/CD8+ double positive T cells have been defined in numerous infections, together with both autoimmune and malignant diseases. The sanctions of CD4+/CD8+ T cells are fully understood, but some studies showed the cytotoxic and the suppressive effects of these cells on cancer. The cytokine profile expression varies in different cancer subtypes, but is biased towards the IL-2, IL-4, IL-5, IL-13 and GM-CSF [11]. The presence of T cells was studied in lymphoma, melanoma, colorectal and breast cancer, but has not been mentioned in neuroblastoma. Our findings might be key to understanding neuroblastoma regression and the immune system response.
Granulomatous areas were associated with the highest immune cell density; a sarcoid granulomatous reaction has previously been reported in NB [12] but is thought to be extremely rare. In the section analysed, there was no associated foreign material, but in other sections granulomas were particularly abundant and there were associated calcifications. As there is no history of core biopsy or disruption of the tumor, it is reasonable to postulate that the calcifications are secondary to the granulomatous inflammation. Therefore, de-novo delayed-type hypersensitivity reaction may have been ongoing prior to excision. It is reasonable to assume this would play a significant role in driving adaptive immunity and eliminating tumors.
5. Conclusion
This technique has allowed us to interrogate the immune response in unprecedented detail and relate this to micro-anatomical niches within the tumor, as well as morphological heterogeneity. We have shown a geographical complexity to the immune response. The nature, extent and distribution of the cellular immune response, attacking the tumor from around the capsule, is definitive evidence that spontaneous regression of NB is an immune-mediated event. In addition, we believe that this analysis captures the dynamic balance of the pro- and anti-tumor immune response. Crucially, it is apparent that this exists in a state of homeostasis across the tumor boundary.
Conflicts of Interest
The authors have no potential conflict of interest; there are no affiliations with or involvement in any organisations or entities with any financial interests.
Consent
Written informed consent for publication of these clinical details and the sample images was obtained from the parents.
Ethical Approval
The ethical approval was gained to stain the sample, sponsor protocol number: RHM CHI1006, IRAS Project ID: 264668.
Author Contributions
Hammouche D: Conception of the work, design of the work, drafting the work, and agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; Easton AS: Acquisition of data, analysis of data, interpretation of data, drafting the work, revising the work critically for important intellectual content, and final approval of the version to be published; Lopez MA: Acquisition of data and analysis of data; Taylor J: Acquisition of data and analysis of data; Marchado M: Acquisition of data and analysis of data; Gray JC: Conception of the work, final approval of the version to be published, agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- H Shimada, S Umehara, Y Monobe, et al. “International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children's Cancer Group.” Cancer, vol. 92, no. 9, pp. 2451-2461, 2001. View at: Publisher Site | PubMed
- S Goto, S Umehara, R B Gerbing, et al. “Histopathology (International Neuroblastoma Pathology Classification) and MYCN status in patients with peripheral neuroblastic tumors: a report from the Children's Cancer Group.” Cancer, vol. 92, no. 10, pp. 2699-2708, 2001. View at: Publisher Site | PubMed
- Takahiro Tsujikawa, Sushil Kumar, Rohan N Borkar, et al. “Quantitative Multiplex Immunohistochemistry Reveals Myeloid-Inflamed Tumor-Immune Complexity Associated with Poor Prognosis.” Cell Rep, vol. 19, no. 1, pp. 203-217, 2017. View at: Publisher Site | PubMed
- G Gorini, M T Ciotti, G Starac, et al. “Fc gamma receptors are expressed on human neuroblastoma cell lines: lack of correlation with N-myc oncogene activity.” Int J Neurosci, vol. 62, no. 3-4, pp. 287-297, 1992. View at: Publisher Site | PubMed
- Joel F G Cohen Solal, Lydie Cassard, Emilie M Fournier, et al. “Metastatic melanomas express inhibitory low affinity fc gamma receptor and escape humoral immunity.” Dermatol Res Pract, vol. 2010, pp. 657406, 2010. View at: Publisher Site | PubMed
- Peter Bankhead, Maurice B Loughrey, José A Fernánde, et al. “QuPath: Open source software for digital pathology image analysis.” Sci Rep, vol. 7, no. 1, pp. 16878, 2017. View at: Publisher Site | PubMed
- Li Ling Lin, Chao Cheng Huang, Chia Ling Wu, et al. “Downregulation of c-Myc is involved in TLR3-mediated tumor death of neuroblastoma xenografts. Laboratory Investigation.” vol. 96, no. 7, pp. 719-730, 2016. View at: Publisher Site | PubMed
- Barbara Hero, Thorsten Simon, Ruediger Spitz, et al. “Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97.” J Clin Oncol, vol. 26, no. 9, pp. 1504-1510, 2008. View at: Publisher Site | PubMed
- Garrett M Brodeur “Spontaneous regression of neuroblastoma.” Cell Tissue Res, vol. 372, no. 2, pp. 277-286, 2018. View at: Publisher Site | PubMed
- K K Matthay “Stage 4S neuroblastoma: what makes it special?” J Clin Oncol, vol. 16, no. 6, pp. 2003-2006, 1998. View at: Publisher Site | PubMed
- Nana H Overgaard, Ji Won Jung, Raymond J Steptoe, et al. “CD4+/CD8+ double-positive T cells: more than just a developmental stage?” J Leukocyte Biol, vol. 97, no. 1, pp. 31-38, 2015. View at: Publisher Site | PubMed
- H Hojo, S Suzuki, A Kikuta, et al. “Sarcoid reaction in primary neuroblastoma: case report.” Pediatr Dev Pathol, vol. 3, no. 6, pp. 584-590, 2000. View at: Publisher Site | PubMed
