Received: Thu 20, Aug 2020
Accepted: Mon 14, Sep 2020
Abstract
Spiradenocarcinoma is a rare skin adnexal neoplasm that may behave aggressively. It is often associated with a benign slow-growing spiradenoma that has undergone malignant transformation, characterised by rapid growth and tenderness. They often present as solitary skin nodules and are classified as low-grade or high-grade tumors. We report a case of a high-grade spiradenocarcinoma in association with a benign spiradenoma, measuring 3.2 x 3 x 2.8 cm, situated on the chest wall of a 63-year-old male. The lesion had been enlarging over 20 years but recently became painful, prompting treatment. Preoperative ultrasound revealed a complex vascular lesion suggestive of a dermoid cyst. Excision of the tumor under general anaesthesia was performed. Histopathology confirmed a high-grade spiradenocarcinoma cleared of margins by 1 mm. Wide local excision of the scar, with a 1 cm margin, confirmed no further malignancy. An FDG PET-CT showed no evidence of distant metastases. The patient recovered well and has had no evidence of tumor recurrence at the 3-month follow-up. Given the rarity of this neoplasm and the paucity of cases in the literature, there is a lack of consensus on treatment. In most cases, surgical excision with adequate margins is the mainstay of treatment, with lymph node dissection reserved for those with suspected or confirmed lymph node metastases.
1. Introduction
Malignant spiradenoma, or spiradenocarcinoma, is a rare tumor seldom reported in the literature. First described by Dabska in 1972 [1], subsequent publications have included mostly case reports and case series with the exception of a meta-analysis performed in 2001 [2], and a retrospective analysis reported in 2013 [3]. Due to its rarity, consensus guidelines do not exist for the management of spiradenocarcinoma. We describe a new case of this rare malignancy.
2. Case Presentation
A 63-year-old Sri Lankan man presented to our General Surgery clinic with a lump on his left chest, which had been present since birth but had increased in size gradually over the past 20 years. The lesion had become painful over the preceding 3 months. On examination, he had an exophytic, non-ulcerated, tender lesion on his left anterior chest with a bluish discolouration. Ultrasound demonstrated a heterogenous, vascular lesion in the subcutaneous tissue to the left of the sternum, measuring up to approximately 3 cm, suggestive of a complex dermoid cyst. Several anechoic areas were seen within the mass, and echogenic areas in the periphery were thought to represent calcifications.
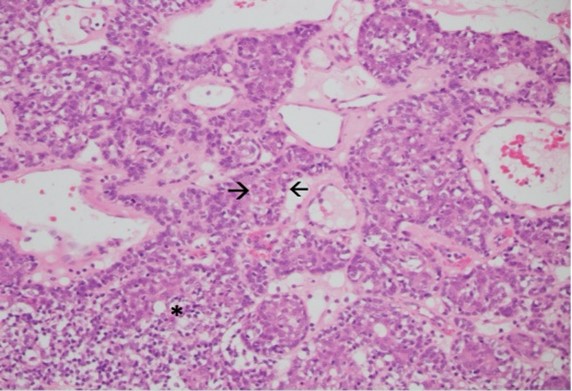
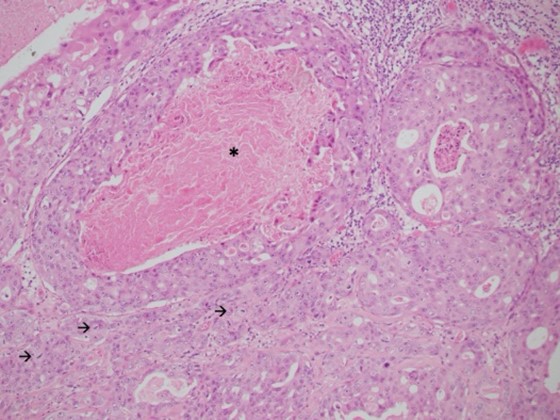
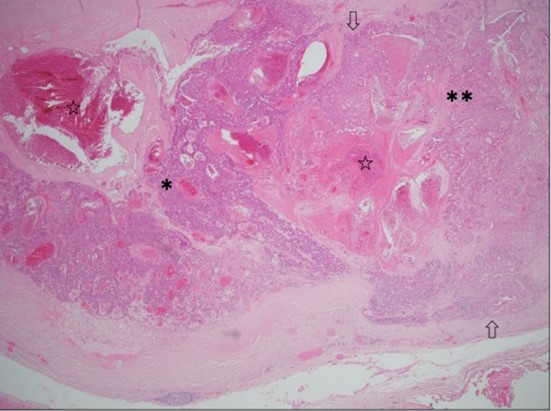
Local excision was performed under general anaesthesia. Pathological evaluation revealed a 32 mm, well-circumscribed nodule within the dermis and subcutis, within 1 mm of the closest margin. Most of the lesion showed classic histological features of spiradenoma comprising anastomosing trabeculae of basaloid cells peripherally and cytologically bland larger cells centrally, set within a loose stroma with scattered small lymphocytes (Figure 1). Markedly ectatic vessels were also present. Adenocarcinoma was present in other areas, comprising large, atypical epithelial cells with vesicular chromatin, macro-nucleoli and abundant amphophilic cytoplasm forming small nests, glands and cribriform structures, some with central comedo necrosis (Figures 2 & 3).
Frequent mitotic figures, including atypical forms, were present. The stroma was variably sclerotic. These findings indicated high-grade adenocarcinoma, not otherwise specified, arising within a spiradenoma. Immunohistochemical staining showed that the adenocarcinomas cells were positive for CK7 and GATA-3, but negative for CK20, oestrogen receptor, TTF-1, CDX-2 and PSA. The consensus of a multidisciplinary team of surgeons and pathologists was for a further excision to achieve a margin of 1 cm or more. Histology from the re-excision did not identify residual in situ or invasive malignancy. An FDG PET-CT showed no evidence of other primary malignancy or metastatic disease. At three-month follow up, the patient had recovered well with no evidence of recurrence.
3. Discussion
Our case describing this rare, aggressive malignancy provides new data that highlights the challenges in diagnosing and managing spiradenocarcinoma. Previous publications consist mostly of case reports and small case series, with one retrospective analysis reported in 2013, containing data from the SEER registry. The majority of patients in that analysis were older than 51 years (72.1%), similar to our patient [3]. Staging has recently been recommended with MRI and FDG PET/CT scanning [4]. Management usually entails primary resection of the lesion. In the 2013 retrospective analysis, 39 (90.7%) of 43 cases underwent surgical resection, but details of lymph node dissection or other biopsies were not provided. Two cases (4.7%) had no surgical or radiotherapy [3].
Adjuvant therapy has been less frequently reported. In a recent case report from 2017, imatinib was given to a 44-year-old female with recurrence and lung metastases; however, the patient died from disease at 15 months follow up [4]. In another case, tamoxifen was administered to a 70-year-old man with lymph node metastasis whose immunohistochemistry demonstrated ER positivity. He was well at 41 months follow up with no evidence of disease [5]. A 5-year survival rate of 76.7% was reported in 2013 [3]. A meta-analysis from 2011 reported a disease-free survival rate of 100% at 33 months’ follow up for patients who underwent surgical resection for non-metastatic disease. However, in view of the risk of metastatic disease, the authors recommended that patients with local disease undergo wide local excision, aiming for surgical margins as recommended for melanoma (in the absence of guidelines specific to spiradenocarcinoma). Lymph node dissection for patients with clinically suspicious or biopsy-proven positive nodes was recommended, based on favourable disease-free survival in six out of seven patients who underwent lymph node dissection for lymph node involvement.
The median survival for 24 patients found to have distant metastasis was 16 months, with no statistically significant difference between patients that underwent surgery alone compared to surgery with adjuvant therapy [2]. Staiger et al. have argued that excision of distant metastases has no therapeutic value, reporting that all cases identified in their literature review with distant metastases died within four months [4]. Regular follow-up should be provided with the aim of detecting recurrence early, enabling surgical resection and potentially preventing distant metastases. Some authors have suggested three-monthly reviews in the year after-treatment, six-monthly in the second year, and annually thereafter. Given the rate of lung and liver metastases, annual chest X-ray and liver function tests have also been recommended [6].
4. Conclusion
There are no consensus guidelines outlining an approach to spiradenocarcinoma. It has been suggested that patients presenting with spiradenocarcinoma undergo an MRI and FDG PET/CT to establish the extent of disease [4]. It has also been recommended that these patients undergo a surgical excision with wide margins, reserving lymph node dissection for those with clinically suspicious or biopsy-confirmed lymph node metastases [2]. Regular follow up has been suggested given the risk of recurrence, including annual chest x-ray and liver function tests [6]. Further research, including retrospective cohort studies and meta-analyses, are required to refine an evidence-based approach to spiradenocarcinoma.
Conflicts of Interest
None.
Funding
None.
REFERENCES
- M Dabska “Malignant transformation of eccrine spiradenoma.” Pol Med J, vol. 11, no. 2, p. 388-396, 1972. View at: PubMed
- Michael T Andreoli, Kamal M F Itani “Malignant eccrine spiradenoma: a meta-analysis of reported cases.” Am J Surg, vol. 201, no. 5, pp. 695-699, 2011. View at: Publisher Site | PubMed
- J Jacob B Avraham, Dana Villines, Vijay K Maker, et al. “Survival after resection of cutaneous adnexal carcinomas with eccrine differentiation: risk factors and trends in outcomes.” J Surg Oncol, vol. 108, no. 1, pp. 57-62, 2013. View at: Publisher Site | PubMed
- Roxane D Staiger, Birgit Helmchen, Claudia Papet, et al. “Spiradenocarcinoma: A Comprehensive Data Review.” Am J Dermatopathol, vol. 39, no. 10, pp. 715-725, 2017. View at: Publisher Site | PubMed
- Imran Mirza, Robert Kloss, Steven C Sieber “Malignant eccrine spiradenoma.” Arch Pathol Lab Med, vol. 126, no. 5, pp. 591-594, 2002. View at: Publisher Site | PubMed
- Basil M Hantash, Joanna L Chan, Barbara M Egbert, et al. “De novo malignant eccrine spiradenoma: a case report and review of the literature.” Dermatol Surg, vol. 32, no. 9, pp. 1189-1198, 2006. View at: Publisher Site | PubMed
