Received: Mon 12, Oct 2020
Accepted: Mon 02, Nov 2020
Abstract
Low grade serous ovarian cancer (LGSOC) accounts for 10% of ovarian cancer cases and is characterised by an early age of onset and an indolent disease course. In contrast to high grade serous ovarian cancer (HGSOC), LGSOC is relatively resistant to cytotoxic chemotherapy and so aggressive surgery is preferred, especially at first presentation. Bevacizumab is effective in advanced ovarian cancer, and several single-institution studies have reported activity in patients with recurrent LGSOC. Bevacizumab is contraindicated in patients with extensive malignant bowel involvement due to the risk of perforation, which can be fatal. We present two patients with advanced LGSOC and extensive bowel serosal disease who safely received combination chemotherapy and bevacizumab in the first line setting and demonstrate that surgery was specifically facilitated by the bevacizumab component of this regimen.
1. Introduction
Low grade serous ovarian cancer (LGSOC) is a rare ovarian cancer subtype, accounting for only 10% of cases. It is histologically, molecularly and genomically distinct from high grade serous ovarian cancer (HGSOC) [1]. LGSOC harbours KRAS, BRAF or ERBB2 variants [2] rather than the ubiquitous TP53 variants and homologous recombination deficiencies frequently seen in HGSOC [3]. Women are typically diagnosed at a younger age (median 55.5 years vs. 62.6 years for HGSOC) and have a more indolent disease course [4]. LGSOC is notoriously chemo-resistant [5, 6], with a response rate of less than 25%, compared to 70% in HGSOC [7].
Primary debulking surgery is the mainstay of initial treatment for epithelial ovarian cancer (EOC) and complete macroscopic (R0) resection significantly improves survival [8]. European Society for Medical Oncology (ESMO) guidelines recommend that all EOC patients with International Federation of Gynaecology and Obstetrics (FIGO) stage II-IV disease receive six cycles of platinum/taxane combination chemotherapy with or without bevacizumab [9]. This recommendation also applies to LGSOC despite its relative chemoresistance. LGSOC commonly (58%) expresses the oestrogen receptor [1]. A recent retrospective analysis of 203 LGSOC patients who had completed primary debulking surgery and adjuvant chemotherapy demonstrated that maintenance treatment with the aromatase inhibitor letrozole significantly extended progression-free survival (PFS) to 64.9 months compared to 26.4 months in patients who were observed [10]. Maintenance therapy with aromatase inhibitors or tamoxifen has now been incorporated into National Comprehensive Cancer Network (NCCN) guidelines. Treatments targeting the MAPK pathway such as the MEK inhibitors selumetinib [11] and trametinib [12] have also demonstrated benefit but as yet have only been explored in recurrent disease.
Bevacizumab is a recombinant humanised monoclonal antibody targeting the vascular endothelial growth factor A ligand (VEGF-A) that is extensively used in the treatment of all histological subtypes of epithelial ovarian cancer (EOC). Randomized phase III clinical trials have investigated its use as first line treatment (GOG218 [9], ICON7 [13]) and in relapsed disease (AURELIA [14], OCEANS [15], GOG213 [16]). When assessing the safety profile of bevacizumab, warnings were given regarding the risks of gastrointestinal wall disruption, including perforation, fistula/abscess formation and surgical wound healing complications such as anastomotic leaks. In the GOG213 study, 15% patients receiving chemotherapy plus bevacizumab experienced gastrointestinal perforation, fistula or abscess compared to 4% in those receiving chemotherapy alone [16]. Bevacizumab is, therefore, generally contraindicated in patients with extensive disease associated with the bowel.
Notably, these studies enrolled patients with all histological subtypes of EOC and did not specify the percentage of patients with a diagnosis of LGSOC. However, small non-randomized retrospective studies have highlighted promising responses to bevacizumab, specifically in LGSOC. One study of 11 patients with recurrent LGSOC treated with bevacizumab either as a single agent (2/11) or in combination with chemotherapy (9/11) saw an overall response rate (ORR = proportion of patients with a partial or complete response) of 55% [17]. In another study, 40 patients with recurrent LGSOC treated with bevacizumab alone (3/40) or in combination with chemotherapy (37/40) had an ORR of 47.5% and a median PFS of 10.2 months [18]. In contrast, in a single centre study of all LGSOC patients treated with single agent bevacizumab, the response rate was only 8% (1/12), but a long median PFS of 48 months was observed, perhaps indicating some anticancer activity [19].
Here we present two patients with advanced LGSOC, treated at two high volume, specialist gynaecologic oncology tertiary referral centres. Both patients had extensive intra-abdominal disease, including large bowel serosal involvement, which prohibited primary cytoreductive surgery. They experienced radiological disease progression during neo-adjuvant chemotherapy but had a marked response following the addition of bevacizumab, which facilitated debulking surgery with complete resection of all macroscopic disease.
Case 1
A 47-year-old female presented to her GP with a 2-month history of abdominal bloating and decreased appetite. She had a past medical history of endometriosis and had undergone a hysterectomy with conservation of ovaries three years previously. Histology was benign. Investigations revealed a raised serum CA125 of 489 kiu/l. Abdominal ultrasound (US) scan showed a large complex cystic mass arising from the right ovary, together with a cystic area on the left side of the pelvis and moderate ascites. A CT scan confirmed bilateral adnexal masses together with large volume omental disease involving the caecum, ascending and sigmoid colon and tumor deposits on the surface of the spleen. She also had a moderate left pleural effusion, but cytology was negative for malignant cells. The radiological stage was FIGO IIIC. The omental biopsy confirmed LGSOC. The specialist multidisciplinary team considered that owing to the extent of serosal bowel disease, surgery would have necessitated a total colectomy; therefore, neoadjuvant chemotherapy was advised.
After 4 cycles of three-weekly carboplatin (AUC 5 based on EDTA renal clearance) and paclitaxel (175mg/m2) chemotherapy CA125 was static at 434 kiu/l (Figure 1). A CT scan of her abdomen and pelvis showed progressive disease, with an increased size of peritoneal deposits and matted loops of bowel, again precluding cytoreductive surgery (Figure 2). Bevacizumab (7.5mg/kg) was added from cycle 5, after which her serum CA125 was seen to fall. She required paracentesis on three occasions between diagnosis and her first cycle of chemotherapy and a further four times during chemotherapy. After introduction of bevacizumab, no further paracentesis was required. A CT scan performed after three cycles of combined carboplatin, paclitaxel and bevacizumab showed a good partial response to therapy with resolution of the omental cake and marked reduction in serosal disease (Figure 2), and CA125 had reduced to 50kiu/l (upper limit of normal =35kiu/l) (Figure 1).
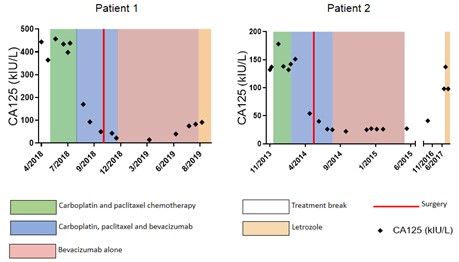
Serum CA125 levels (kiu/l) over time. Green shading indicates chemotherapy alone; blue shading: chemotherapy together with bevacizumab; pink shading: bevacizumab alone; white: treatment break; orange: letrozole and red line: date of surgery.
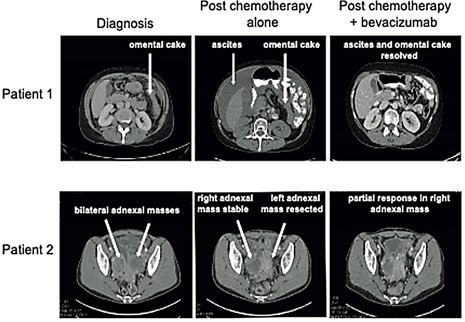
CT images of Patient 1 and Patient 2 at diagnosis, following chemotherapy and again following chemotherapy given in combination with bevacizumab. Specific disease areas are labelled. Upfront debulking surgery (prior to treatment with either chemotherapy or bevacizumab) was attempted in Patient 2 but surgery was limited to a left oophorectomy owing to the extent of disease.
The patient then underwent debulking surgery, including a bilateral salpingo-oopherectomy, anterior resection, splenectomy, omentectomy and abdominal and pelvic peritonectomy with no macroscopic residual disease. She received one final cycle of combined carboplatin, paclitaxel and bevacizumab followed by maintenance single agent bevacizumab for a further 12 cycles with no adverse effects. Unfortunately, at this point (eight months from completion of chemotherapy) her CA125 began to rise (75 kiu/L from a nadir of 14 kiu/L). CT scan confirmed progressive disease compared to a post-operative CT scan, and she commenced letrozole therapy.
Case 2
A 35-year-old female presented to her GP with a 3-week history of abdominal pain and constipation. She had no medical or gynaecology history. US revealed bilateral adnexal masses and serum CA125 was 132 kiu/l. On CT scan, the bilateral pelvic masses were adherent to the pelvic side wall with involvement of the appendix, rectosigmoid and bladder, in addition to a thick omental cake. The radiological stage was FIGO IIIC. An attempt at primary cytoreductive surgery was abandoned when miliary disease was seen covering the surface of the liver and both hemi-diaphragms, as well as extensive serosal nodules overlying the bowel. The left adnexal mass was excised during surgery and histology confirmed LGSOC.
She was enrolled in the ICON8 clinical trial and was randomized to receive three-weekly carboplatin (AUC 6) and paclitaxel (175 mg/m2). After three cycles there was no reduction in serum CA125 (Figure 1) and CT showed disease progression, with a new mass within the left adnexal bed and enlarged peritoneal deposits. She was subsequently withdrawn from the trial and bevacizumab (7.5mg/kg) was added to the chemotherapy. After three cycles of triple therapy, her serum CA125 had fallen from 151 to 54 kiu/l, and a CT scan showed a good partial response to treatment, with resolution of the diaphragmatic and serosal disease (Figure 2). Importantly, the patient also reported a reduction in abdominal pain and bowel symptoms. She was subsequently able to have a complete surgical resection including total abdominal hysterectomy, bilateral salpingo-oopherectomy, rectosigmoid colectomy with end colostomy formation, appendicectomy and removal of serosal nodules, with no residual macroscopic disease. Of note, the same surgeon performed both operations, showing that a difference in surgical skill could not have accounted for the successful complete macroscopic resection during the second operation. She completed 18 cycles of bevacizumab, as per standard of care, with no adverse effects. She eventually experienced CA125 progression according to Gynaecologic Cancer Intergroup Committee (GCIC) criteria. CT confirmed disease relapse 26 months after completion of bevacizumab and she was commenced on letrozole treatment.
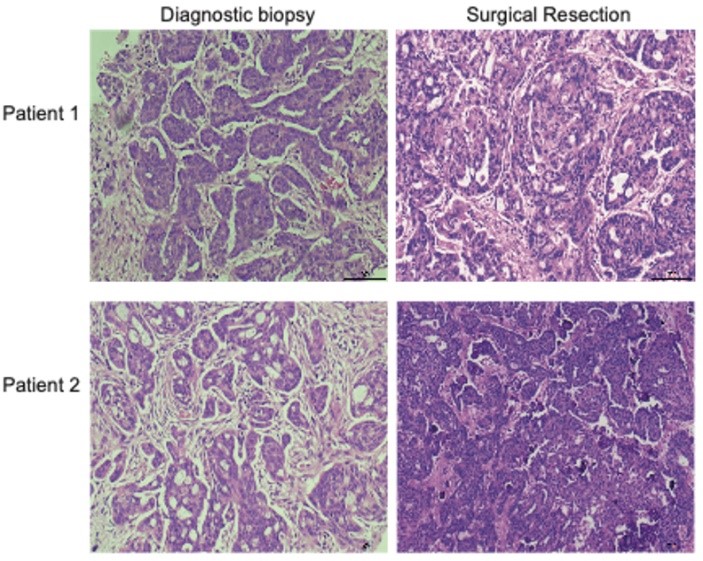
Haematoxylin and eosin (H&E) stained slides from diagnostic omental biopsies and surgically resected omentum were examined in both patients, and pathological chemotherapy response score (CRS) was assessed (Figure 3). We previously defined this scoring system in omental metastasis of HGSOC, where a CRS of 1 indicates minimal pathological response to chemotherapy, and CRS of 3 indicates near resolution of disease [20]. We have also shown that CRS correlates with patient survival in HGSOC, but this system is not usually applied to LGSOC. Surgical omental sections in our two patients both revealed a CRS of 1, with large areas of persistent viable tumor in examined sections. This lack of pathological response to chemotherapy is consistent with the radiological progression experienced by both patients when carboplatin and paclitaxel were given alone and further supports the key role of bevacizumab in effecting the radiological responses that ultimately enabled successful surgery (Figure 2).
2. Conclusion
In conclusion, we present two patients with advanced LGSOC and extensive intra-abdominal disease, including widespread bowel involvement that prohibited primary cytoreductive surgery. In both cases, there was radiological disease progression following standard treatment with carboplatin and paclitaxel chemotherapy. Following the addition of bevacizumab, both patients experienced symptomatic benefit with reduction in pain, bowel symptoms and the need for ascitic drainage. This was reflected in marked radiological reductions in disease volume, which ultimately enabled surgery. Remarkably, bowel serosal disease was improved such that all macroscopic disease could be excised, and only short-length bowel resections were required.
Complete macroscopic resection is known to be the most important prognostic factor in the management of LGSOC [8], even in advanced disease (FIGO III/IV), and so standard practice includes resection of large volumes of viable tumor. We propose that bevacizumab can assist biochemical, radiological and clinical response and specifically facilitate surgery in chemotherapy-resistant, inoperable LGSOC. Importantly, bevacizumab was safe in these two LGSOC patients with extensive bowel involvement. Whilst bevacizumab has been studied in LGSOC previously, published studies are extremely sparse and all are retrospective. There are none comparing bevacizumab alone to bevacizumab plus chemotherapy, and no studies investigating the value and safety of its use in the neoadjuvant setting. Interestingly, in the only study of single agent bevacizumab in LGSOC, the response rate appeared to be lower [19]; it may be that in LGSOC the main benefit is gained from bevacizumab and chemotherapy being given in combination. Both patients experienced durable symptomatic improvement once bevacizumab was added, but evaluation of any potential impact on survival would require a larger randomized study. In view of the recent study from Gershenson et al. [10], it would be interesting to compare maintenance therapy with letrozole and bevacizumab following first line treatment. In conclusion, further evaluation of bevacizumab in the neo-adjuvant setting, particularly in patients that have had no response to conventional chemotherapy, is recommended.
Acknowledgements
ML was supported by a Cancer Research UK Clinician Scientist Fellowship (C41405/A13034) and a Cancer Research UK Advanced Clinician Scientist Fellowship (C41405/A19694). HH was supported by a CRUK Clinical Research Training Fellowship (S_3398) and GEW was supported by Barts and The London Charity Clinical Research Training Fellowships (MGU0370 and MGU0469). We thank the two patients whose cases are presented here and who provided their informed consent under the ethics of the Barts Gynae Tissue Bank (REC: 15/EE/0151). Finally, we thank the tissue bank staff and the clinical teams involved in these women’s care.
Author Contributions
H. Hockings: Devised methodology and conducted investigation, data curation and formal analysis, wrote original draft and reviewed & edited the final manuscript. G.E. Wood: Contributed to writing original draft, reviewed & edited the final manuscript. J. McDermott: Investigation and formal analysis, visualization of data, reviewed & edited the final manuscript. P. Narayanan: Investigation and formal analysis, visualization of data. A. Hameeduddin: Investigation and formal analysis, visualization of data. R.E. Miller: Supervised study. Contributed to methodology and data curation. Reviewed & edited the final manuscript. M Lockley: Conceptualization and funding acquisition, data curation and formal analysis, devised methodology and conducted investigation, project administration and supervision, wrote original draft, reviewed & edited the final manuscript.
Conflicts of Interest
REM and ML both report personal fees from Roche, outside the submitted work. The authors have no other conflicts of interest to disclose.
REFERENCES
- Kurman RJ, Shih IM “The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded.” Am J Pathol, vol. 186, no. 4, pp. 733-747, 2016. View at: Publisher Site | PubMed
- Singer G, Oldt R, Cohen Y, et al. “Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma.” J Natl Cancer Inst, vol. 95, no. 6, pp. 484-486, 2003. View at: Publisher Site | PubMed
- Bell D, Berchuck A, Birrer M, et al. “Integrated genomic analyses of ovarian carcinoma.” Nature, vol. 474, no. 7353, pp. 609-615, 2011. View at: Publisher Site | PubMed
- Plaxe SC “Epidemiology of low-grade serous ovarian cancer.” Am J Obstet Gynecol, vol. 198, no. 4, pp. 459.e8-459.e9, 2008. View at: Publisher Site | PubMed
- Gershenson DM, Sun CC, Bodurka D, et al. “Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant.” Gynecol Oncol, vol.114, no. 1, pp. 48-52, 2009. View at: Publisher Site | PubMed
- Grabowski JP, Harter P, Heitz F, et al. “Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase.” Gynecol Oncol, vol. 140, no. 3, pp. 457-462, 2016. View at: Publisher Site | PubMed
- Ledermann JA, Raja FA, Fotopoulou C, et al. “Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.” Ann Oncol, vol. 24, no. 6, pp. vi24-vi32, 2013. View at: Publisher Site | PubMed
- Bois A Du, Reuss A, Pujade Lauraine E, et al. “Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO).” Cancer, vol. 115, no. 6, pp. 1234-1244, 2009. View at: Publisher Site | PubMed
- Burger RA, Brady MF, Bookman MA, et al. “Incorporation of bevacizumab in the primary treatment of ovarian cancer.” N Engl J Med, vol. 365, no. 26, pp. 2473-2483, 2011. View at: Publisher Site | PubMed
- Gershenson DM, Bodurka DC, Coleman RL, et al. “Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum.” J Clin Oncol, vol. 35, no. 10, pp. 1103-1111, 2017. View at: Publisher Site | PubMed
- Farley J, Brady WE, Vathipadiekal V, et al. “Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study.” Lancet Oncol, vol. 14, no. 2, pp. 134-140, 2013. View at: Publisher Site | PubMed
- Gershenson D, Miller A, Brady W, et al. “A Randomized Phase II/III Study to Assess the Efficacy of Trametinib in Patients with Recurrent or Progressive Low-Grade Serous Ovarian or Peritoneal Cancer.” Ann Oncol, vol. 30, no. 5, pp. v851-v934, 2019.
- Perren TJ, Swart AM, Pfisterer J, et al. “A Phase 3 Trial of Bevacizumab in Ovarian Cancer.” N Engl J Med, vol, 365, no. 26, pp. 2484-2496, 2011. View at: Publisher Site | PubMed
- Pujade Lauraine E, Hilpert F, Weber B, et al. “Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial.” J Clin Oncol, vol. 32, no. 13, pp. 1302-1308, 2014. View at: Publisher Site | PubMed
- Aghajanian C, Blank SV, Goff BA, et al. “OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer.” J Clin Oncol, vol. 30, no. 17, pp. 2039-2045, 2012. View at: Publisher Site | PubMed
- Coleman RL, Brady MF, Herzog TJ, et al. “Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial.” Lancet Oncol, vol. 18, no. 6, pp. 779-791, 2017. View at: Publisher Site | PubMed
- Grisham RN, Iyer G, Sala E et al. “Bevacizumab Shows Activity in Patients With Low-Grade Serous Ovarian and Primary Peritoneal Cancer.” Int J Gynecol Cancer, vol. 24, no. 6, pp. 1010-1014, 2014. View at: Publisher Site | PubMed
- Schmeler KM, Tao X, Sun CC, et al. “Encouraging responses with bevacizumab in recurrent low-grade serous ovarian cancer.” J Clin Oncol, vol. 28, no. 15, pp. e15503, 2010.
- Rose PG, Mahdi H, Jernigan A, et al. “Activity of Bevacizumab in Patients with Low-Grade Serous Ovarian Carcinoma.” Int J Gynecol Cancer, vol. 26, no. 6, pp. 1048-1052, 2016. View at: Publisher Site | PubMed
- Böhm S, Faruqi A, Said I, et al. “Chemotherapy response score: Development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma.” J Clin Oncol, vol. 33, no. 22, pp. 2457-2463, 2015. View at: Publisher Site | PubMed
