Received: Mon 22, Jun 2020
Accepted: Thu 09, Jul 2020
Abstract
Pregnancy-associated pulmonary embolism remains the leading cause of direct maternal mortality globally. We will present a case of a young lady who was one month post-delivery via normal vaginal delivery. She presented with pulmonary edema and her clinical examination was consistent with severe mitral stenosis. Notably, her previous and recent pregnancies were uneventful. The key question was: why is there a sudden deterioration? “Why now?”. This led to an industrious search of a precipitant for her presentation; a diagnosis of severe rheumatic mitral stenosis complicated by pulmonary edema precipitated by a pulmonary embolism was made. The patient later had a valve replacement with good outcomes.
1. Case Report
Patient presentation: A 30-year-old G3P3 woman was referred to our unit 1 month after having had an uncomplicated vaginal delivery of a healthy baby. She was well until 2 days before referral, when she developed sudden onset of shortness of breath, which progressed rapidly over the subsequent 24 hours, to the point where she was short of breath at rest with PND. The accompanying symptoms included pleuritic chest pain and a non-productive cough. She denied palpitations, haemoptysis, fever, a productive cough, and lower limb pain or swelling.
er prior pregnancies were uneventful, culminating in the vaginal delivery of full-term babies. She was HIV negative, and there was no history of previous surgeries. There was no known history of childhood rheumatic fever or congenital heart disease. Except for injectable contraceptives, she was not using any medications. She neither smoked nor consumed alcohol, and she denied recreational drug use. On arrival, she was mildly febrile with temp 37.6ocelsius, the blood pressure was 105/69mmHg, heart rate was 125 beats per minute, and respiratory rate was 30 breaths per minute. Her oxygen saturation was 99% on a non-rebreather mask, a significant improvement from of 64% on room air. The physical examination was notable for a normal jugular venous pressure, a cardiac apex which was non-displaced with a palpable first heart sound, which was loud on auscultation. A diastolic murmur with pre-systolic accentuation was appreciated at the apex with the patient lying on the left side. At the left parasternal border, there was a pan-systolic murmur of tricuspid regurgitation. The respiratory system revealed bilateral crackles, and the abdomen was normal with no hepatomegaly, splenomegaly or ascites.
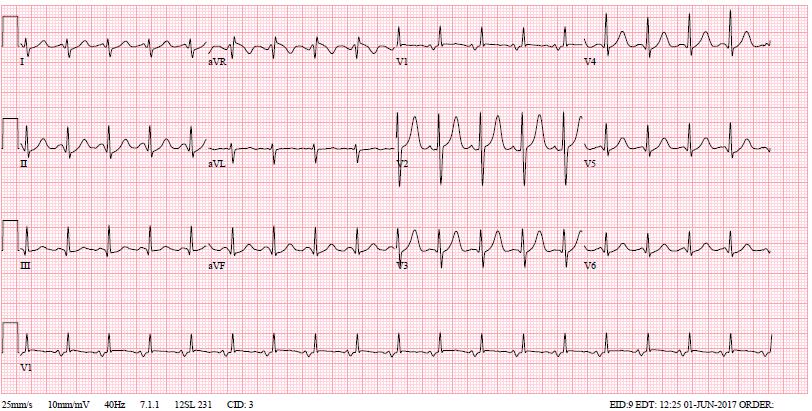
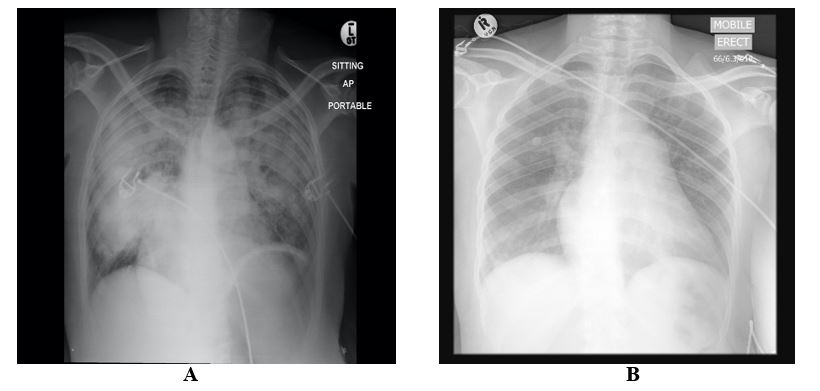
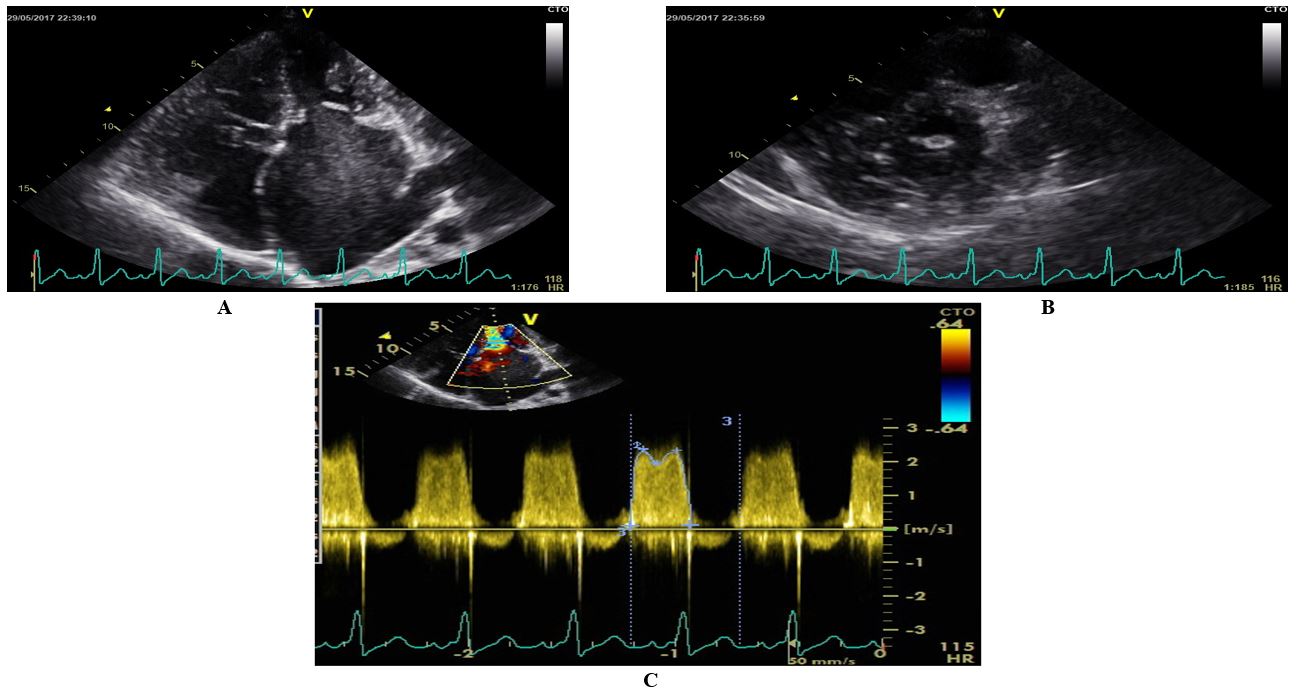
Her electrocardiogram (ECG) is illustrated in (Figure 1). Notable findings included sinus tachycardia, right axis deviation (900), left atrial hypertrophy and a dominant R in V1. The laboratory studies showed an elevated white cell count of 30 x 109/L with the predominance of neutrophils (87%). Her electrolytes and renal function were normal. Her chest X-ray was notable for a bat’s wing pattern of bilateral airspace opacification indicative of pulmonary edema (Figure 2). Her echocardiography is presented in (Figure 3). It confirmed the clinical diagnosis of severe rheumatic mitral stenosis with a mean gradient of 16 mmHg, left atrial dilatation with echo ‘smoke,’ normal left ventricular dimensions with normal LV systolic function. The Wilkin’s score was 9/16.
Clinical Course: Given the diagnosis of tight mitral stenosis complicated by acute pulmonary edema the patient received intravenous diuresis aggressively over the first 24 hours while she was continued on face mask oxygen.
Rheumatic severe mitral stenosis is a post-inflammatory fibrostenotic disease in which the left atrial pressure is chronically elevated. Therefore, an acute pulmonary edema presentation should always stimulate a search for precipitants. Because this was one month postpartum and was out of the window of cardiovascular vulnerability, pregnancy as a precipitant of her acute pulmonary edema was considered less likely.
Pneumonia was also considered unlikely despite the mild fever and leucocytosis, given the acuteness of the illness and lack of other characteristic symptoms such as sputum production. Tachyarrhythmia was considered a possibility, but the patient did not have palpitations, and there was no ECG and telemetry evidence of a rhythm disturbance. Finally, given the hypercoagulability of pregnancy and the hormone contraceptives she was using, she was treated empirically for an acute pulmonary embolism until she was well enough to either confirm or refute the diagnosis with appropriate testing. On day 3, after she had clinical resolution of her pulmonary edema, she went for a 99m Tc lung ventilation-perfusion scan which revealed a perfusion mismatch in the entire right lower lung lobe as well as a matched perfusion defect in the superior basal segment of the left lung with perfusion being affected to a greater extent. Ventilation and perfusion to the lungs were inhomogeneous and more pronounced in the perfusion study. These findings were interpreted as high probability of a large PE.
2. Discussion
Pregnancy-associated pulmonary embolism remains the leading cause of direct maternal mortality globally, partly due to the fact that the clinical diagnosis of venous thromboembolism (VTE) in pregnancy is usually obscured by the maternal physiological changes that happen in pregnancy that may mimic symptoms and signs of VTE [1, 2]. VTE complicates 1 in 1000 pregnancies and is approximately 10 times more common in pregnant women compared with non-pregnant women [3, 4]. For example, in a study undertaken in Limpopo, South Africa , a total of 14,685 live births and 232 maternal deaths were reported over the five-year period of the study, resulting in a maternal mortality ratio of 1579 per 100 000 live births, 3 % (n=7) were due to pulmonary embolism [5]. Pregnancy-related VTE is one of the 10 most important potentially preventable causes of maternal death in South Africa (SA) [6].
During pregnancy, VTE events occur with equal distribution across all three trimesters [7]. By 20 weeks’ gestation, more than half of women affected will have had a VTE event [8]. The risk of venous thromboembolism is also increased postnatally by approximately 20-fold compared to normal age-matched controls. Although the risk extends until at least 12 weeks into the postnatal period [9], most thromboembolic events occur in the first 3 weeks after delivery [9, 10].
While the association between valvular heart disease and pulmonary embolisms risk of VTE has only recently been confirmed [11], the association between mitral stenosis and pulmonary infarction was recognized as early as the 1930s [12]. The recognition and diagnosis of acute pulmonary embolus in patients with valvular heart disease and heart failure may be particularly difficult given the potential masking of “typical symptoms and signs” and difficulties distinguishing these from those of the underlying cardiac disease and heart failure [13]. In pregnant patients and those with valvular heart disease who present with pulmonary emboli, the predictors of a poor outcome are not dissimilar to other patients with pulmonary embolus [14]. Patients who present with hemodynamic instability are usually at increased risk of death [15]. The outcomes of the condition depend on early recognition and adequate therapy being instituted during the early stages. Early recognition and treatment have been reported to significantly decrease mortality rates [15, 16]. It is recommended that if there is clinical suspicion of thromboembolism, treatment can be commenced pending confirmation of diagnosis [17].
The title of the article “Why now?” is a practical question that clinicians should aim to answer whenever there is a sudden deterioration of an otherwise “quiescent” mitral stenosis. When this occurs, a useful short list of precipitants to think about and rule out clinically or with appropriate testing is provided in (Table 1) below. In this particular patient, it was the pursuit of the answer to this question that allowed for confirmation of the diagnosis and treatment with both short term anticoagulant therapy and long-term warfarin, thus significantly reducing the likelihood of recurrence with even more dire consequences.
|
Cardiac precipitants |
Non-cardiac precipitants |
|
New tachyarrhythmia (a fib most common) |
Infection, e.g., Pneumonia, UTI |
|
Infective endocarditis |
Acute or occult blood loss, e.g., GIT bleed |
|
Pulmonary embolism |
Pregnancy (most vulnerable at 24-36 weeks) |
|
Acute ischemia (e.g., thromboembolism to coronary artery) |
Thyrotoxicosis |
3. Conclusion
We have presented a patient with a background of severe rheumatic mitral stenosis complicated by pulmonary edema precipitated by a pulmonary embolism in the early postpartum period. The case served as a very useful reminder of the importance of looking for potential precipitants in patients with an unexplained acute deterioration. In this particular incidence, the fact that she had not decompensated during pregnancy and the suddenness of her symptoms prompted a thorough search for pulmonary embolus, which was treated successfully with appropriate therapy. The patient was scheduled for valve replacement therapy after allowing a prolonged bonding period with her newborn baby daughter.
Conflicts of Interest
None.
Funding
None.
REFERENCES
- Knight M, Tuffnell D, Kenyon S, et al. “Saving lives, improving mothers' care: Surveillance of maternal deaths in the UK 2011-13 and lessons learned to inform maternity care from the UK and Ireland. Confidential enquiries into maternal deaths and morbidity 2009-13.” 2015.
- Simcox LE, Ormesher L, Tower C, et al. “Pulmonary Thrombo-Embolism in Pregnancy: Diagnosis and Management.” Breathe (Sheff), vol. 11, no. 4, pp. 282-289, 2015. View at: Publisher Site | PubMed
- Anderson FA, Wheeler HB, Goldberg RJ, et al. “A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: the Worcester DVT Study”. Arch Intern Med, vol. 151, no. 5, pp. 933-938, 1991. View at: Publisher Site | PubMed
- White RH “The epidemiology of venous thromboembolism.” Circulation, vol. 107, no. 1, pp. I4-I8, 2003. View at: Publisher Site | PubMed
- Ntuli ST, Mogale M, Hyera FL, et al. “An investigation of maternal mortality at a tertiary hospital of the Limpopo province of South Africa.” South Afr J Infectious Dis, vol. 32, no. 2, pp. 73-76, 2017. View at: Publisher Site
- Moodley J, Fawcus S, Pattinson R “Improvements in maternal mortality in South Africa.” South Afr Med J, vol. 108, no. 3, pp. 4-8, 2018.
- Bourjeily G, Paidas M, Khalil H, et al. “Pulmonary embolism in pregnancy.” Lancet, vol. 375, no. 9713, pp. 500-512, 2010. View at: Publisher Site | PubMed
- Gherman RB, Goodwin TM, Leung B, et al. “Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy.” Obstet Gynecol, vol. 94, no. 5, pp. 730-734, 1999. View at: Publisher Site | PubMed
- Kamel H, Navi BB, Sriram N, et al. “Risk of a thrombotic event after the 6-week postpartum period.” N Engl J Med, vol. 370, no. 14, pp. 1307-1315, 2014. View at: Publisher Site | PubMed
- Jacobsen AF, Skjeldestad FE, Sandset PM “Incidence and Risk Patterns of Venous Thromboembolism in Pregnancy and Puerperium--A Register-Based Case-Control Study.” Am J Obstet Gynecol, vol. 198, no. 2, pp. 233.e1-233.e7, 2008. View at: Publisher Site | PubMed
- Sørensen HT, Horvath Puho E, Lash TL, et al. “Heart Disease May Be a Risk Factor for Pulmonary Embolism Without Peripheral Deep Venous Thrombosis.” Circulation, vol. 124, no. 13, pp. 1435-1441, 2011. View at: Publisher Site | PubMed
- Levine HB, White PD “Pulmonary Infarction Complicating Severe Disease of The Mitral Valve.” Arch Intern Med, vol. 60, no. 1, pp. 39-50, 1937.
- Raja AS, Greenberg JO, Qaseem A, et al. “Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians.” Ann Intern Med, vol. 163, no. 9, pp. 701-711, 2015. View at: Publisher Site | PubMed
- Goldhaber SZ, Visani L, De Rosa M “Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER).” Lancet, vol. 353, no. 9162, pp.1386-1389, 1999. View at: Publisher Site | PubMed
- Charlebois D “Early recognition of pulmonary embolism: the key to lowering mortality.” J Cardiovasc Nurs, vol. 20, no. 4, pp. 254-259, 2005. View at: Publisher Site | PubMed
- Barcan A, Tarta D, Tarta C “Clinical Update. Clinical Presentations of Pulmonary Embolism in the Emergency Department.” J Cardiovas Emergencies, vol. 3, no. 3, pp. 133-137, 2017. View at: Publisher Site
- Thomson AJ, Walker ID, Greer IA “Low-molecular-weight heparin for immediate management of thromboembolic disease in pregnancy.” Lancet, vol. 352, no. 9144, pp. 1904, 1998. View at: Publisher Site | PubMed
