Received: Mon 06, Jul 2020
Accepted: Fri 17, Jul 2020
Abstract
Objective: The objective of this study was to summarize the prevalence and the available management strategies for cardiogenic shock in the setting of severe aortic stenosis and their outcome.
Introduction: Aortic stenosis (AS) is the most common valvular heart disease in older adults. Cardiogenic shock (CS) is a medical emergency, and while management for these two conditions has evolved over the years, little is known about the prevalence, management, and mortality of AS in combination with CS.
Methods: We performed a systematic review to identify studies that included patients with severe AS presenting with CS using EMBASE, MEDLINE, and Scopus. Primary outcomes included in-hospital, 30-day, and 1-year mortality. Additional outcomes included procedure-related complications. We registered the study protocol at PROSPERO (CRD42018112245).
Results: We included a total of 10 studies representing 338 patients. In-hospital mortality ranged from 43% to 77% in patients treated with Balloon Aortic Valvuloplasty (BAV), 0% in patients who underwent surgical valve replacement (SAVR), and 11% and Transcatheter aortic valve replacement (TAVR). In patients undergoing BAV, 30-day mortality varied between 50% and 55%, and between 19% and 33% in those treated with TAVR. There was limited evidence on the use of Mechanical Circulatory Support (MCS).
Conclusion: AS presenting with CS is a rare but fatal condition, and no consensus exists regarding management strategy. Time to intervention on the valve is critical. Valve replacement through either SAVR or TAVR had better outcomes compared to BAV alone. There is limited evidence on MCS as a management strategy in patients with AS complicated by CS.
1. Introduction
Aortic stenosis (AS) constitutes the majority of valvular heart disease in developed countries, and its prevalence increases with age. In those 65 years or older, the prevalence is 25% and rises to 50% above 85 years of age [1-3]. AS is typically characterized by a slowly progressing fibro-calcific process leading to left ventricular outflow obstruction [4].
Further, acute LV and circulatory decompensation can happen as a consequence of severe AS [5, 6]. The management of severe AS presenting with cardiogenic shock (CS) is challenging and requires management of both pump failure and valvular obstruction. The combination of pump failure and outlet obstruction often poses a dilemma as to the type and timing of valvular intervention, as many patients presenting in CS are poor surgical candidates due to multi-organ dysfunction. There is limited knowledge about the optimal treatment of shock with concomitant severe AS [7]. We aimed to understand the prevalence of CS as a consequence of acutely decompensated severe AS, determine the frequency, utilization, and outcomes related to different interventions for patients with CS due to decompensated severe AS, and assess outcomes with the use of mechanical support devices in patients with severe AS who present in CS.
2. Materials and Methods
We prospectively registered this review in the International Prospective Register of Systematic Reviews PROSPERO (Link 1; protocol registration number: CRD42018112245). Data reporting in this review are following the preferred reporting items for systematic review and meta-analyses (PRISMA) statement [8]. We performed our study from publicly available data of published literature. We conducted a comprehensive search of databases (Ovid MEDLINE, Ovid EMBASE, and Scopus) from the inception of available data through October 2018. The search strategy was designed and carried out by a medical librarian (K.F.) with initial input from the study’s first author. The abstracts found by the search were screened for eligibility by two independent authors (M.E., M.T.). The two authors further screened all retrieved references of the included studies for potential additional studies. The study’s inclusion and conflicts were resolved by consensus between 2 reviewers. The search terms are available in (Supplementary Table 1).
We included English language retrospective and prospective cohort studies as well as clinical trials that included adult patients. Our patient population of interest needed to have severe AS with concomitant CS. We excluded abstracts presented in scientific meetings due to a lack of full clinical data. We further excluded case reports and review articles. If the same group of authors published multiple studies, we only included the largest study with relevant patient population and outcomes. Two authors (M.E., J.F.) abstracted the data from articles independently. A third author (C.B) was the reference for settling discrepancies regarding data extraction after discussion between all three authors. Abstracted data included the following: enrollment period, study design, patients’ age, and gender; country of origin, surgical risk stratification scores including Society of Thoracic Surgery risk score (STS), EuroScore and Log EuroScore, relevant echocardiographic parameters, type of intervention, procedural mortality and complications, and overall mortality.
The quality assessment for the included studies was done independently by three reviewers (M.E, C.B, and J.F.) using the NIH Quality Assessment Tool for Observational Studies (Link 2). The quality assessment tool provides a guide for systematically assessing the risk of different types of bias in the study and the internal validity of the study results. This tool is composed of a list of items concerning key methodological points and specific criteria to be fulfilled for each item. The tool does not employ a numeric scale; instead, authors rate each study as either good, fair, or poor according to the estimated risk of bias (low, intermediate, or high), which depends on the number and types of unfulfilled criteria. Three authors (M.E, C.B, and J.F) independently applied this tool to each of the studies. For each question, the answer was either yes, no, cannot determine, or not applicable. Then, the overall quality of each study, as well as the discordances, were finalized by consensus between the three authors.
Due to the heterogeneity of both exposure and outcomes assessed by the studies included in this systematic review, we were unable to perform a meta-analysis. In particular, the paucity of studies and the incomplete information provided in the published manuscripts prevented the identification of meaningful subgroups suitable for meta-analysis; therefore, we summarized the available evidence using qualitative systematic review methodology.
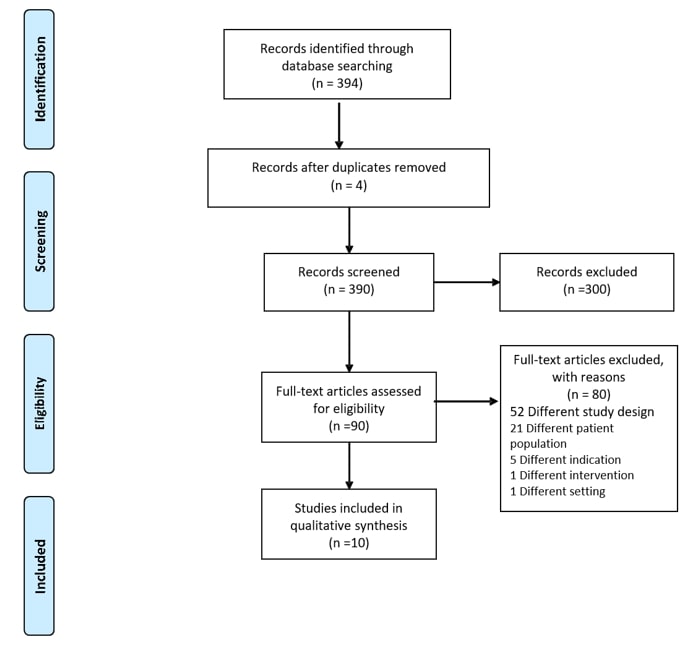
TABLE 1: Study Characteristics.
|
Author/ Year |
Country |
Data collection period |
Population |
Design |
N |
Prevalence of (AS+CS) |
Mortality end-point |
Age (Mean ± SD) |
Male % |
|
Moreno 1994 [9] |
United States |
1986 - 1993 |
Single-center, consecutive patients with severe AS referred for BAV (N=310) presenting with CS. |
Retrospective |
21 |
6.70% |
In-hospital mortality, 1-year mortality |
74 ± 3 |
47% |
|
Buchwald 2001 [10] |
Germany |
1989 - 1998 |
Single-center, consecutive patients with severe AS referred for BAV and presenting with CS. |
Retrospective |
14 |
- |
In-hospital, 1-year mortality |
74.1± 11.4 |
50% |
|
Aksoy 2011 [11] |
United States |
2006 - 2009 |
Single-center, consecutive patients with severe aortic stenosis who received IABP. (N=285) as a bridge to BAV or AVR and presenting with CS |
Retrospective |
35 |
12.20% |
In-hospital mortality |
73.5 ± 9.5 |
76% |
|
D’Ancona 2012 [12] |
Germany |
2008 - 2011 |
Single-center study of consecutive patients with severe AS referred for transapical TAVI (N=358) and presenting with CS. |
Prospective |
21 |
5.80% |
30-day, |
74.5± 11.1 |
32% |
|
|
|
|
|
|
1-year mortality |
||||
|
Saia 2013 [13] |
Italy |
2000 - 2010 |
Single-center study of consecutive patients with severe AS referred for BAV (N=415) presenting with CS. |
Retrospective |
23 |
5.50% |
In-hospital, 1-year mortality |
70± 12.9 |
56.5% |
|
Frerker 2016 [14] |
Germany |
2008 - 2013 |
Single-center study of consecutive patients with severe AS referred for TAVI (N=771) presenting with CS. |
Retrospective |
27 |
3.50% |
In-hospital, 30-day, 1-year mortality |
78 ± 9 |
44.4% |
|
Debry 2018 [15] |
France |
2011 - 2016 |
Multi-center study of consecutive patients with severe AS referred for BAV (N=293) and presenting with CS |
Retrospective |
31 |
10.5%** |
1-Year mortality |
77.3 ± 8.6 |
84% |
|
Bongiovanni 2018 [16] |
Germany |
2009 - 2015 |
Multi-center study of patients referred for TAVI or BAV (N=3000), with suitable anatomy in cardiogenic shock or refractory pulmonary edema. |
Retrospective |
141 |
4.70% |
Peri-procedural mortality, 30-day mortality |
80.3 ± 3.1 |
70% |
|
Eugene 2018 [17] |
France |
2008 - 2016 |
Single-center study of consecutive patients with severe aortic stenosis and either CS or refractory pulmonary edema. |
Retrospective |
17 |
|
In-hospital mortality |
79 ± 9 |
70% |
|
Karatolios 2018 [18] |
Germany |
2014 - 2016 |
Single-center study of consecutive patients with severe AS and CS who underwent BAV then Impella 2.5 placement. |
Retrospective |
8 |
|
Procedural mortality, 30-day mortality |
78.3 ± 9.6 |
62.5% |
** Study by Debry, they did not report the actual number of the entire screened population; instead, they mentioned that the included patients were “around 15%” of the entire population.
3. Results
We included three hundred and ninety-one references in the initial sample and screened three hundred and ninety abstracts after the removal of four duplicates. We retrieved ninety full-text studies for possible inclusion. Of the retrieved studies, only ten full-text manuscripts representing a total of 351 patients met the inclusion criteria (Figure 1 & Table 1) [9-18]. All studies were single-center studies mostly carried out in the United States [9, 11] and Europe [10, 12-18], published between 1994 and 2018, with the longest period of observation was ten years. All studies were retrospective cohort studies, except for one prospective study by D’Ancona et al. [12]. Most subjects were elderly male (32% to 84%), ranging between 70 to 80 years old, as presented in (Table 1). All the included patients in ten studies had severe AS defined as aortic valve area (AVA) < 1 cm2, however only five studies reported peak gradients, most reported mean gradients and four reported average mean gradients < 40 mmHg, which is consistent with low flow low gradient aortic stenosis. All studies included patients with moderate to severely reduced ejection fraction and reported comorbidities such as coronary artery disease and chronic kidney disease, shown in (Table 2).
TABLE 2: Patients’ Characteristics at Baseline.
|
Author/Year |
Risk Score |
Echocardiographic characteristics |
Comorbidities |
||||||
|
STS |
Log EuroScore |
AVA (cm)2 |
Peak Gradient |
Mean Gradient |
LVEF (%) |
History of CAD% |
CKD |
||
|
Moreno 1994 [9] |
* |
* |
0.48±0.04 |
* |
49 ± 4 |
29 ± 3 |
38% |
43% |
|
|
Buchwald 2001 [10] |
* |
* |
0.38±0.09 |
80.0±19.1 |
* |
* |
36% |
* |
|
|
1Aksoy 2011 [11] |
* |
Medical management: |
0.64±0.11 |
67 ± 26.8 |
39.8±16.8 |
32.6±13.9 |
36% |
36% |
|
|
16.8 ± 2.2 |
|||||||||
|
Surgical management: |
|||||||||
|
15 ± 3 |
|||||||||
|
D’Ancona 2012 [12] |
50.8 ± 28.1 |
73.1 ± 18.9 |
0.6±0.02 |
* |
* |
26 ± 13.1 |
62% |
* |
|
|
Saia 2013 [13] |
- |
39.5 ± 20.1 |
0.6±0.2 |
66.6±19.4 |
42 ± 13 |
39.9±14.9 |
52.2% |
91.3% |
|
|
Frerker 2016 [14] |
* |
60.4 ± 21.1 |
0.8±0.2 |
42.2±24.3 |
37 ± 16.5 |
39.5±15.4 |
70.4% |
63% |
|
|
Debry 2018 [15] |
25.9 ± 11.9 |
45.8 ± 13.5 |
0.65±0.15 |
- |
38.6 ± 15 |
29 ± 13 |
38% |
55% |
|
|
Bongiovanni 2018 [16] |
eTAVI |
* |
37.7 ± 18.1 |
0.59±0.05 |
* |
* |
* |
65.2% |
* |
|
eBAV |
35.3 ± 20.8 |
0.60±0.02 |
64.3% |
||||||
|
eBAV then elective TAVI |
25.9 ± 13.9 |
0.67±0.04 |
59.4% |
||||||
|
Eugene 2018 [17] |
38 ± 13 |
70 ± 13 |
0.57±0.2 |
* |
41 ± 17 |
27 ± 11 |
53% |
29% |
|
|
Karatolios 2018 [18] |
39 ± 11.8 |
71 ± 13.7 |
0.7 ± 0.2 |
69.6±20.4 |
38.8 ± 8.7 |
35.1 ±1.8 |
50% |
100% |
|
1Euroscore II system was used. *Missing data
STS: Society of Thoracic Surgery Risk Score; AVA: Aortic Valve Area; LVEF: Left Ventricular Ejection Fraction; CAD: Coronary Artery Disease; CKD: Chronic Kidney Disease; eTAVI: emergency Transthoracic Aortic Valve Implantation; eBAV: emergency Balloon Aortic Valvuloplasty.
Definitions of cardiogenic shock and hemodynamic deterioration were variable among the studies, but the commonly used criteria were tissue hypoperfusion/ impaired organ function, the presence of catecholamines, and systolic blood pressure of £ 90 mmHg. The presence of pulmonary congestion and decreased creatinine clearance were used only in a few studies, as seen in (Supplementary Table 2). Of the included ten studies, only 7 reported the total number of patients evaluated in order to identify the subgroup of interest (severe AS and CS) [9, 11-16]. Among the studied populations, the prevalence of CS associated with severe AS ranged from 3.5% to 12%. Four studies included CS patients with severe AS undergoing balloon valvuloplasty (BAV) [9, 10, 13, 15]. Prevalence in the aforementioned studies is summarized in (Table 1). In addition to BAV, some of the included studies in our review investigated valve replacement strategies, including SAVR [11] and TAVR [12, 16].
In-hospital mortality varied across studies depending on the intervention and population that was studied. When used as a rescue intervention for CS in the setting of severe AS, BAV in-hospital mortality remained high across studies, ranging from 43% to 77% [9, 10, 13, 15, 17, 18]. Comparing surgical valve replacement to medical treatment, Aksoy et al., demonstrated that in-hospital mortality in patients treated with IABP and medical therapy was 91%, compared to 100% survival for patients who received surgical valve replacement. There was not a significant difference in Euroscore between the two groups (16.8 ± 2.2 vs. 15 ± 3.16; p = NS) [11]. Only one study reported an in-hospital mortality of 11% in patients with severe AS in CS for transfemoral TAVR and 54% for those with a transapical approach [14]. Of the ten included studies, four studies reported 30-day mortality [12, 14-16, 18]. In patients who underwent BAV, the mortality at 30 days was 50 to 55% [15, 18]. In two separate studies, the reported mortality rates following aortic valve replacement were generally lower [12, 14], 19% following a transapical TAVR through a mini-thoracotomy and 33% 30-day mortality using a transfemoral TAVR. Pneumonia and sepsis accounted for six out of nine reported deaths at 30 days rather than cardiovascular complications.
TABLE 3: Procedural Characteristics, Complications, Outcomes.
|
Author/ Year |
Procedural characteristics |
Overall mortality |
Procedural complications |
||||||||
|
Type |
Mechanical support |
Valve replacement % (N) |
In-hospital
% (N) |
30-day
%(N) |
1-year
%(N) |
Procedural mortality %(N) |
Vascular complications %(N) |
CVA
%(N) |
Aortic insufficiency (N) |
||
|
Moreno 1994 [9] |
BAV |
- |
19% (4) |
43% (9) |
- |
67% (14) |
9% (2) |
24% (5) |
9.5% (2) |
1 |
|
|
Buchwald 2001 [10] |
BAV |
- |
14% (2) |
71% (10) |
- |
71% (10) |
0% (0) |
21% (3) |
- |
2 |
|
|
Aksoy 2011 [11] |
SAVR |
IABP |
44% (11) |
In-Hospital Mortality: |
7% (1) * |
12% (3) |
- |
- |
|||
|
Medical management (11): 91% (10) |
|||||||||||
|
Mechanical management (14): 7% (1)**. |
|||||||||||
|
Total: 44% (11) |
|||||||||||
|
D’Ancona 2012 [12] |
Transapical TAVI |
CPB1 |
- |
- |
19% (4) |
54% (11) |
0% (0) |
- |
0% |
0 |
|
|
Saia 2013 [13] |
BAV |
- |
4.4% (1) |
56.6% (13) |
NA |
70.7% (16) |
0% |
2.2% (1) |
0% |
2 |
|
|
Frerker 2016 [14] |
TAVR |
CPB2 |
- |
11% (3) |
33% (9) |
40.7% (13) |
4% (1) |
33% (9) |
3.7% (1) |
1 |
|
|
Derby 2018 [15] |
BAV |
- |
19% (6) |
51.6% (16) |
55% (17) |
77% (24) |
6% (2) |
0% |
3.2% (1) |
1 |
|
|
Bongiovanni 2018 [16] |
eBAV |
- |
- |
- |
33% (39) |
- |
20.3% (24) |
12.9% (15) |
0% |
19 |
|
|
eTAVR |
- |
- |
- |
23.8% (5) |
- |
8.7% (2) |
21.7% (5) |
8.7% (2) |
1 |
||
|
eTAVR after eBAV |
- |
27% (32) |
- |
21.9% (7) |
- |
9.4% (3) |
15.6% (5) |
6.3% (2) |
4 |
||
|
Eugene 2018 [17] |
BAV |
- |
- |
48% (8) |
- |
- |
0% |
- |
- |
1 |
|
|
Karatolios 2018 [18] |
BAV |
Impella 2.5 |
37% (3) |
50% (4) |
50% (4) |
- |
0% |
12.5% (1) |
0% |
- |
|
147.6% (10 of 21)
225.9% (7 of 27)
* Intra-operative death was during a BAV procedure.
** Deceased patient received BAV without AVR
(-) Missing data
BAV: Balloon Aortic Valvuloplasty; TAVR: Trans-Catheter Aortic Valve Replacement; SAVR: Surgical Aortic Valve Replacement; eBAV: emergency Balloon Aortic Valvuloplasty; TAVI: Transapical Aortic Valve Implantation; CPB: Cardiopulmonary Bypass; IABP: Intra-Aortic Balloon Pump.
Bongiovanni et al. compared the outcome of 3 cohorts: emergency TAVR, emergency BAV, and elective TAVR following emergency BAV. They found that emergency BAV had the highest mortality amongst the three groups (33%) followed by emergency TAVR (23.8%), and elective TAVR following emergency BAV (21.9%). However, the outcomes reported were not statistically different across groups, as shown in (Table 3). In a multivariate analysis, the only independent variable associated with fatal outcome in patients with AS and CS treated with BAV was a duration of shock > 48 hours. Among 14 patients with severe AS and CS, four patients survived to hospital discharge while the remaining ten did not. All patients who survived were intervened upon within the 48 hours window (8 to 18 hours), while those who did not had a delayed intervention (48 hours to 8 days) [10]. This finding was also demonstrated by Debry et al. [15], who concluded that delayed BAV (>48 hours from starting inotropic agent) predicted mortality and CS recurrence at one year (90% vs. 59%; p = 0.01).
Although mechanical support is a well-established intervention for cardiogenic shock, the data is sparse on the use of mechanical support in severe AS with CS. While Aksoy et al. showed that IABP placement was feasible in 25 patients [11], those who were only supported by IABP without valve intervention had a very high in-hospital mortality (10/11). Conversely, those who underwent valve replacement survived hospitalization except for one patient who received BAV but did not undergo AVR. There was only one study using an Impella 2.5 for mechanical support, which found that it was feasible after BAV in patients with severe AS and CS [18]. In-hospital mortality for this series of 8 patients was 50%, which is consistent with other reports evaluating outcomes after BAV in this patient population. We used specific questions from the NIH quality assessment tool for observational cohort studies to assess the internal validity as well as the risk of bias of each study. The questions and answers are demonstrated in (Supplementary Tables 3A & 3B, respectively). We rated five studies as good quality [9, 12, 14, 15, 19], three studies as fair [11, 16, 18], and the remaining two studies as poor [10, 17]. All included studies were retrospective except for the study by D’Ancona et al. As an inherent limitation for observational studies, none of the authors reported a sample size justification, in addition, since the incidence of the condition of interest was low it led to small sample sizes.
Significant concerns about the methods used by Buchwald et al. were heterogeneity in the timing of valvular intervention and the management of shock among patients treated with BAV, as well as the lack of quantitative information on the definition of aortic stenosis. With regards to the study by Eugene et al. and Bongiovanni et al., both studies included patients with refractory pulmonary edema secondary to severe AS as an equivalent to CS. Further, the allocation of a patient in each intervention group was at the discretion of the operator, potentially introducing selection bias. Four [11, 15, 17, 18] of the ten studies did not adjust for potential confounders in their statistical analysis, although Derby et al. justified this based on the small sample of patients recruited. Finally, questions 8, 9, and 10 were not applicable across all the included studies.
4. Discussion
In this systematic review, we found that while CS is not a typical presentation for severe AS, there is a limited amount of data on its prevalence. Most studies are retrospective observation studies without control cohorts, with the exception of one study. Relevant studies varied in terms of clinical definitions, patient characteristics, and treatments. This heterogeneity was mainly due to the different clinical settings in which the authors conducted their studies. Additionally, there was no uniform definition of cardiogenic shock across studies; thus, we were not able to draw a concrete conclusion about CS prevalence in severe AS.
BAV was the most widely used intervention for AS in the emergency setting, and mortality rates were comparable between the different studies that utilized BAV. Compared to BAV, aortic valve replacement was found to have better in-hospital and 30-day mortality outcomes as an emergency intervention. Medical management without valvular intervention showed dire outcomes with an in-hospital mortality of 91%. While mechanical circulatory support was noted to be feasible in this niche patient population, there was no clear evidence in the available literature that it improves mortality outcomes.
Although the studies in this review included patients from a variety of settings, authors concluded that severe AS was the main factor precipitating circulatory decompensation in these patients. The majority of patients were high-risk for surgery in the studies that utilized BAV as an intervention [9, 10, 13, 15-18]. Patients were consistently older with a mean age > 70 years and had at least one comorbidity that made them unfit to pursue a valve replacement strategy, according to the authors. The older age of this patient population, as well as the presence of comorbidities could explain why BAV instead of valve replacement was utilized in the majority of available studies in the literature. The studies we included in this review were from 1994 to 2018, and the mortality rate did not seem to change when considering BAV, ranging from 43% to 71%. The 30-day mortality rates reported were comparable to those reported by the IABP-SHOCK II trial, which was approximately 40% [5].
Comparing BAV vs. TAVR as an emergent rescue procedure, Bongiovanni et al. [16] retrospectively assessed the outcomes of emergent BAV (eBAV) vs. emergent TAVR (eTAVR) in patients with acutely decompensated AS. The 30-day mortality was lower for eTAVR compared to eBAV, (23.8% vs .33%, P=NS). However, since the study was retrospective by nature, there was no randomization of patients to either arm of comparison. Another major limitation of this study was the lack of clear distinction between CS and NYHA IV with refractory pulmonary edema when assigning the patients to either intervention, which might either over or underestimate the mortality benefit of both interventions. Two studies investigating outcomes following TAVR [12, 14], found a 30-day mortality after transapical TAVR of 19% [12], mostly attributed to multi-organ failure, conversely 33% after femoral TAVR [14], reportedly due to pneumonia and sepsis rather than cardiovascular death. Though there is no comparison of BAV to TAVR, the cohort of patients included in these studies have high surgical risk. In the two aforementioned studies, included patients with high surgical risk with a Log Euroscore of 60.4 (21.1) and 73.1 (18.9), respectively, whereas those undergoing BAV had Log EuroScore between 39.5-71 [17, 18]. This indicates that valve replacement strategy can potentially have better outcomes than lone BAV in this niche population even in the presence of higher surgical risk scores with the caveat that surgical risk scores do not accurately capture the full clinical picture of these patients on admission.
Surgical aortic valve replacement in the acute setting of cardiogenic shock was performed in one study arm by Aksoy et al., after a bridge of circulatory support on IABP [11]. Of the 25 patients included in this study, 14 received mechanical intervention on the valve (11 SAVR, 4 BAV, 1 received both). All patients who had valve intervention survived to hospital discharge, while the remaining patients who were only supported by IABP and medically managed, all but one died in hospital. The calculated surgical risk for both arms in this study (valve intervention vs. supportive management) was not statistically different since surgical mortality for the group that received supportive measures was 63.07 ± 17.29% vs. 50.32 ± 23.20%. Thus, this discrepancy in mortality between the two groups is likely attributed to the relief of outflow obstruction by valve replacement, since surgical risk scores were similar, and all patients received mechanical support.
Despite the paucity of data, one of the essential signals emerging from 2 of the included studies is the importance of the timing of intervention on the aortic valve. Albeit in a small sample, the studies by Buchwald et al. [10] and Debry et al. [15] concluded that the sooner the intervention, the better the chances of survival. It is essential to underline that both studies were mainly assessing the outcomes of BAV. This observation is in keeping with prior reports assessing the timing of mechanical support use in cardiogenic shock [20, 21], as earlier restoration of tissue perfusion can prevent irreversible end-organ damage. However, it remains unclear whether the suggested 48 hours window by the two studies is, in fact the one associated with the best outcomes. The question remains whether an earlier intervention would be preferred or if a longer time window would still have favorable outcomes as long as valve replacement is considered for patients in whom prompt intervention was not feasible.
Cardiac unloading using mechanical circulatory support devices in order to restore and maintain tissue perfusion early improved outcomes in patients with CS [20-22]. In our review, the studies that discussed the use of mechanical circulatory support for CS management, whether IABP by Aksoy et al. [11], or Impella 2.5 by Karatolios et al. [18], were focused on feasibility and did not have a control group for outcome comparison. While both studies showed improved cardiac hemodynamics after insertion, we were unable to conclude mortality benefits from their use because of the confounding valve intervention, which is likely the intervention that impacted survival. Further, there are also unanswered questions of whether concomitant valve replacement and or mechanical support at the time of BAV or a delayed intervention would be a better management strategy.
The presence of severe AS in patients presenting with CS carries noteworthy mortality and poses unique management challenges. In the 2018 AATS/ACC/SCAI/STS expert consensus on the decision pathway for TAVR, the combination of systolic failure and severe AS carries a poor prognosis [23]. Currently, experts make decisions on how to address the combination of pump failure and valvular obstruction and in which timeframe, is made on a patient to patient basis. Such decisions are likely affected by the operators’ and the facility’s comfort level with different types of interventions without any clear pathway guidelines.
In summary, to our knowledge, this is the first systematic review addressing this exceptionally high-risk population. We have presented a variety of interventions and outcomes, highlighting the complexity and lack of well-developed evidence about this condition. Accurate documentation and prospective registries of patients presenting with decompensated severe AS are necessary to conclude a better idea about their risk profile. Given the paucity of data in this area, the data we have explored in this systematic review should be carefully considered by physicians and surgeons in how they select patients for interventions. Ultimately, a prospective randomized control trial for severe AS patients presenting with CS may be the only definitive way to establish the superiority of one intervention over another.
5. Conclusion
Severe AS complicated by CS is a challenging situation with poor prognosis and relatively scarce data on outcomes. There is no consensus on the optimal treatment and timing of strategy for patients with severe AS presenting in CS. Further studies are needed to establish the best management pathways for severe AS and cardiogenic shock.
Funding
None.
Disclosures
Dr. Lissa Sugeng: Cannon Medical: research grant, speaker’s bureau; Siemens Healthineers: research grant, speaker’s bureau, Advisory Board; Philips Healthcare: research grant, Advisory Board; Hitachi: equipment grant, speaker's bureau. Other authors: No disclosures.
REFERENCES
- Stewart BF, Siscovick D, Lind BK, et al. “Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study.” J Am Coll Cardiol, vol. 29, no. 3, pp. 630-634, 1997. View at: Publisher Site | PubMed
- Cosmi JE, Kort S, Tunick PA, et al. “The risk of the development of aortic stenosis in patients with "benign" aortic valve thickening.” Arch Intern Med, vol. 162, no. 20, pp. 2345-2347, 2002. View at: Publisher Site | PubMed
- Coffey S, Cox B, Williams MJ “The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis.” J Am Coll Cardiol, vol. 63, no. 25, pp. 2852-2861, 2014. View at: Publisher Site | PubMed
- Lindman BR, Clavel M, Mathieu P, et al. “Calcific aortic stenosis.” Nat Rev Dis Primers, vol. 2, pp. 16006, 2016. View at: Publisher Site | PubMed
- Thiele H, Zeymer U, Neumann F, et al. “Intraaortic balloon support for myocardial infarction with cardiogenic shock.” N Engl J Med, vol. 367, no. 14, pp. 1287-1296, 2012. View at: Publisher Site | PubMed
- Hollenberg SM, Kavinsky CJ, Parrillo JE “Cardiogenic shock.” Ann Int Med, vol. 131, no. 1, pp. 47-59, 1999. View at: Publisher Site | PubMed
- Levy B, Bastien O, Karim B, et al. “Experts’ recommendations for the management of adult patients with cardiogenic shock.” Ann Intensive Care, vol. 5, no. 1, pp. 52, 2015. View at: Publisher Site | PubMed
- Moher D, Liberati A, Tetzlaff J, et al. “Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.” Open Med, vol. 62, no. 3, pp. 123-130, 2009. View at: PubMed
- Moreno PR, Jang IK, Newell JB, et al. “The role of percutaneous aortic balloon valvuloplasty in patients with cardiogenic shock and critical aortic stenosis.” J Am Coll Cardiol, vol. 23, no. 5, pp. 1071-1075, 1994. View at: Publisher Site | PubMed
- Buchwald AB, Meyer T, Scholz K, et al. “Efficacy of balloon valvuloplasty in patients with critical aortic stenosis and cardiogenic shock--the role of shock duration.” Clin Cardiol, vol. 24, no. 3, pp. 214-218, 2001. View at: Publisher Site | PubMed
- Aksoy O, Yousefzai R, Singh D, et al. “Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support.” Heart, vol. 97, no. 10, pp. 838-843, 2011. View at: Publisher Site | PubMed
- D'Ancona G, Pasic M, Buz S, et al. “Transapical transcatheter aortic valve replacement in patients with cardiogenic shock.” Interact Cardiovasc Thorac Surg, vol. 14, no. 4, pp. 426-430, 2012. View at: Publisher Site | PubMed
- Saia F, Marrozzini C, Ciuca C, et al. “Emerging indications, in-hospital and long-term outcome of balloon aortic valvuloplasty in the transcatheter aortic valve implantation era.” EuroIntervention, vol. 8, no. 12, pp. 1388-1397, 2013. View at: Publisher Site | PubMed
- Frerker C, Schewel J, Schlüter M, et al. “Emergency transcatheter aortic valve replacement in patients with cardiogenic shock due to acutely decompensated aortic stenosis.” EuroIntervention, vol. 11, no. 13, pp. 1530-1536, 2016. View at: Publisher Site | PubMed
- Debry N, Kone P, Vincent F, et al. “Urgent balloon aortic valvuloplasty in patients with cardiogenic shock related to severe aortic stenosis: time matters.” EuroIntervention, vol. 14, no. 5, pp. e519-e525, 2018. View at: Publisher Site | PubMed
- Bongiovanni D, Kühl C, Bleiziffer S, et al. “Emergency treatment of decompensated aortic stenosis.” Heart, vol. 104, no. 1, pp. 23-29, 2018. View at: Publisher Site | PubMed
- Eugene M, Urena M, Abtan J, et al. “Effectiveness of Rescue Percutaneous Balloon Aortic Valvuloplasty in Patients With Severe Aortic Stenosis and Acute Heart Failure.” Am J Cardiol, vol. 121, no. 6, pp. 746-750, 2018. View at: Publisher Site | PubMed
- Karatolios K, Chatzis G, Luesebrink U, et al. “Impella support following emergency percutaneous balloon aortic valvuloplasty in patients with severe aortic valve stenosis and cardiogenic shock.” Hellenic J Cardiol, vol. 60, no. 3, pp. 178-181, 2018. View at: Publisher Site | PubMed
- Saia F, Marzocchi A, Marrozzini C, et al. “Emergency balloon aortic valvuloplasty in patients with critical aortic stenosis presenting with cardiogenic shock.” Ital Heart J, vol. 6, no. 5, pp. 420-423, 2005. View at: PubMed
- Gul B, Bellumkonda L “Usefulness of Intra-aortic Balloon Pump in Patients With Cardiogenic Shock.” Am J Cardiol, vol. 123, no. 5, pp. 750-756, 2019. View at: Publisher Site | PubMed
- Basir MB, Schreiber TL, Grines CL, et al. “Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock.” Am J Cardiol, vol. 119, no. 6, pp. 845-851, 2017. View at: Publisher Site | PubMed
- Pieri M, Sorrentino T, Oppizzi M, et al. “The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock.” J Interv Cardiol, vol. 31, no. 6, pp. 717-724, 2018. View at: Publisher Site | PubMed
- Writing Committee Members, Bavaria JE, Tommaso CL, et al. “2018 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document: Operator and institutional recommendations and requirements for transcatheter aortic valve replacement: A joint report of the American Association for Thoracic Surgery, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons.” J Thorac Cardiovasc Surg, 2018. View at: Publisher Site | PubMed
